Prof Thomas Jaki at the Medical and Pharmaceutical Statistics Research Unit, Lancaster University outlines the benefits of adaptive clinical trials
The development of new medicinal products and health technologies is time-consuming and expensive. For pharmaceutical products, it is estimated that it takes 10-15 years to develop a novel compound and costs several hundred million pounds on average. The reason for the long duration is that even after a potentially useful compound has been identified, the product needs to undergo pre-clinical animal studies, first-in-man studies and a series of clinical trials addressing different questions such as safety, dosing, and efficacy. The largest contributor to both time and cost are confirmatory (Phase III) clinical trials that often involve thousands of patients with a follow-up period frequently lasting years. Recently only about 50% of confirmatory clinical trials have been able to show that the treatment under investigation has a good enough risk-benefit trade-off to achieve a license by a regulatory authority. At the same time, only around 20% of phase II clinical trials are successful in showing an improvement in the primary endpoint of the study.
The reasons for these unsuccessful trials are thought to be the taking forward of treatments that should have been abandoned earlier, and insufficient precision when determining the optimal dose, assessing safety, or when determining the patient population to be studied further.
One of the fundamental concepts of traditional clinical trials is that, once the study has been designed and recruitment started, no changes to the design are allowed and no analyses of the study data that require knowledge about treatment allocation by the study team are typically allowed until all patients have been recruited and observed in the study. This means that, for example, even if the data would already clearly show that the novel treatment is not promising, further patients will be exposed to this potentially harmful treatment.
One way to overcome this inherent dilemma is adaptive clinical trials. The US FDA defines an adaptive clinical trial as: “A study that includes a prospectively planned opportunity for modification of one or more specified aspects of the study design and hypotheses based on analysis of data (usually interim data) from subjects in the study”. In other words, an adaptive design allows decisions about the study to be made on the basis of the study data while the trial is still ongoing. This allows early decisions about the utility of the intervention under study but also allows other changes to improve the properties of the design.
Typically, only a small number of design changes are permitted – after all, these designs are not a remedy for poor planning. Analyses of the data that inform decisions to alter the design are at prospectively planned time points called interim analyses. Although ideally the interim analyses are conducted in a blinded manner, the nature of the potential adaptations often necessitates unbinding. In either case, it is crucial that the validity and integrity of the study remains intact.
trial design
Adaptive designs are ethical
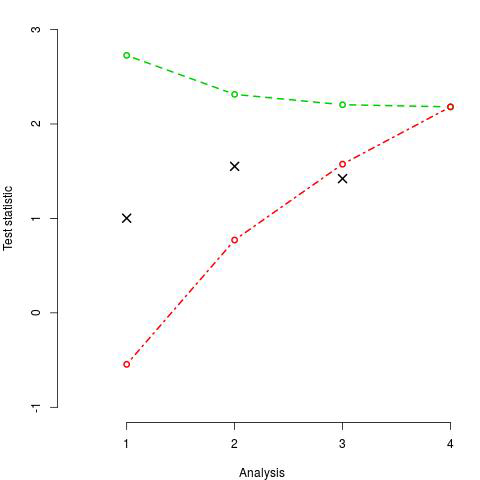
One of the most common adaptive designs are (group-) sequential design. These designs allow stopping the trial early either because results so far are not promising or because the advantage of the treatment under investigation is already established. Specifically, they include a number of interim analyses at each of which a test statistic quantifying the advantage of the experimental treatment over control is found. If the test statistics is below a lower bound, the trial is stopped for lack of benefit as the treatment does not look promising. If the test statistic exceeds an upper bound, the trial is stopped for proven efficacy. Otherwise further patients are recruited into the study. Figure 1 illustrates such a design and we find that no decision is made at the first 2 analyses, while the treatment is deemed not sufficiently promising at the third and hence the trial terminated.
This example clearly shows why adaptive trials are ethical: In a traditional trial, the decision that the treatment is not promising would have been made later and hence additional patients would have been exposed to a treatment that has no additional benefit (but potentially worse safety) than the current standard. More generally, we do expect that the number of patients in the study is smaller for a sequential design compared to a traditional design which means that we expect to stop treatments that are not promising earlier or identify promising treatment quicker.
Adaptive designs are efficient
The group-sequential design above is also efficient as a decision about the utility of a treatment can be made quicker. Multi-arm multi-stage designs, are another highly efficient adaptive design.
Traditionally multiple treatments are evaluated, in separate trials initially, and then one is taken forward for definitive testing (Figure 2a). A multi-arm multi-stage design (Figure 2b) combines the screening of treatments with the confirmatory stage in one trial thereby removing the time gap between the 2 different phases of development. Moreover, they compare multiple novel treatments against a common control group reducing the number of patients on the control treatment and hence the total sample size.
The Medical and Pharmaceutical Statistics Research Unit at Lancaster University develops and evaluates novel statistical methods of study design (such as adaptive designs) and tailors the methods to the needs of specific trials.
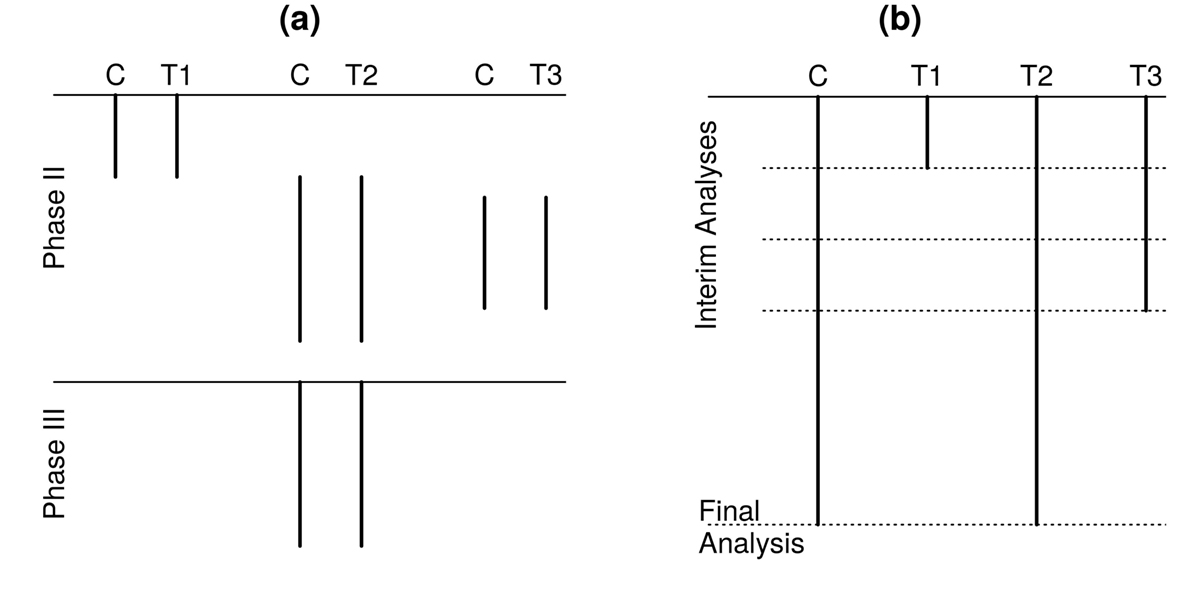
and a multi-arm multi-stage design (b)
Prof Thomas Jaki
Professor of Statistics
Medical and Pharmaceutical Statistics
Research Unit
Lancaster University
Tel: +44 (0)1524 59 23 18
mps@lancaster.ac.uk
Please note: this is a commercial profile











