Sean E. Lawler PhD from Brigham and Women’s Hospital, Harvard Medical School, sheds light on overcoming the barriers when it comes to brain cancer therapeutics
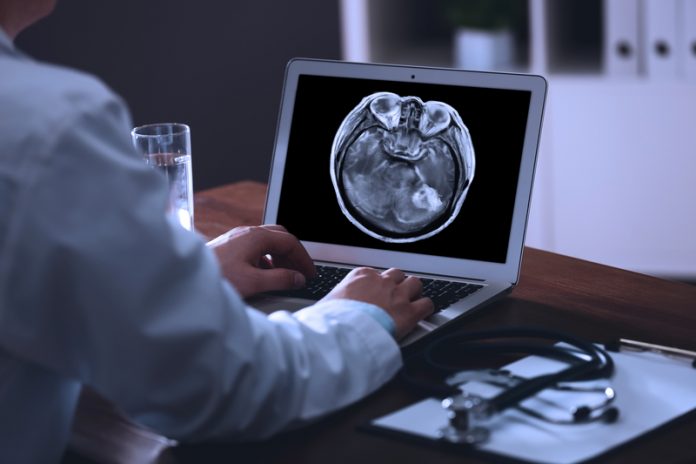
Brain cancer represents one of the most daunting challenges in oncology. The term “brain tumour” encompasses a diverse group of cancer types that grow in the brain, some of which are exceedingly rare. Their location brings a unique set of challenges; sophisticated brain imaging technology is required to identify brain cancer and surgery must be performed with the utmost care and skill. Furthermore, most cancer drugs are rendered ineffective by a uniquely specialised blood vessel structure – the blood-brain barrier (BBB) – which prevents them from entering the brain.
Glioblastoma – what is it? How is it treated?
Of all brain tumours, glioblastoma is the most notorious. It arises without warning and confers a terrifying prognosis; untreated, the patient will decline within months. Even with the best treatment available, most glioblastoma patients will not survive beyond two years of their diagnosis.
A well-established clinical protocol is used to treat glioblastoma. At the beginning of the patient’s journey, the neurosurgery team will carefully remove as much of the tumour tissue as possible. This is analysed in the neuropathology lab for accurate diagnosis, and specialised molecular tests guide subsequent clinical decision making. The patient then moves to the care of the neuro-oncology and radiotherapy teams who administer a course of chemotherapy and irradiation, and responses are monitored by radiological brain imaging. Unfortunately, glioblastoma inevitably returns, and at this point can be more aggressive and is resistant to treatment. Again, the neurosurgeon may be required to remove the regrown tumour, and further experimental therapies may be tested. This disease is now called recurrent glioblastoma, and there is little that can be done to stop it at this time. This dreadful outcome occurs despite the best efforts of clinicians and scientists. Clinical trials with a multitude of approaches have so far failed to improve patient outcomes beyond the incremental.
In better news, the last 20 years have seen an unparalleled explosion in our knowledge of the genetic events that drive cancer. We are also in the midst of a revolution in our understanding of cancer at the cellular level, and how tumour/host ecosystems work. There have been major breakthroughs using immunotherapy to re-educate our own immune systems to destroy some types of tumour which were previously incurable. But not glioblastoma – which has learned very well how to hide from immune cells which may otherwise kill them. Understanding how to manipulate immune responses in the brain to target glioblastoma is currently a major focus of basic scientific studies.
But with any therapy, we still face the challenge of crossing the BBB and then safely targeting glioblastoma cells scattered throughout the brain. Several exciting approaches are under investigation – for example, clinical trials are in progress using focused ultrasound to improve drug delivery by temporarily breaking the BBB. Other approaches try to exploit the biology of BBB to sneak drugs through, Trojan Horse style.
Nonetheless, without some type of cancer cell specificity — a “search and destroy” component — many drugs will fail due to toxicity because of damage to the delicate structures and neuronal connections in our brain that make us who we are and enable us to function. We can, therefore, imagine a “dream drug” for glioblastoma which would be able to cross the BBB and specifically target tumour cells, delivering a toxic payload and leaving everything else untouched. Using in vitro BBB models and a pipeline of drug synthesis, one of the goals of my own lab is to design modular drugs which can be delivered to the patient, cross the BBB, bind selectively to tumour cells, and deliver effective drugs. These early-stage studies are under active investigation and funded by the U.S. National Cancer Insitute.
In another scenario, which bypasses the BBB completely, a therapeutic drug can be applied during surgery. This type of “local delivery” is also used in clinical trials of oncolytic viruses. These are engineered human (or animal) viruses which can infect and kill tumour cells. Oncolytic viruses also alert the immune system to the tumour, potentially overcoming local immunosuppression, “uncloaking” the tumour and opening the door for precise tumour cell killing by the patients’ own immune system.
Genetics and biology of glioblastoma in the lab
These exciting approaches are being driven by scientists studying the genetics and biology of glioblastoma in the lab. We now have analytical tools to identify features linked to therapeutic response and resistance in patient tumours and their biofluids. Another critical lab activity is in pre-clinical studies, using tumour cells from patients and mouse models of glioblastoma. In these models, glioblastoma cells are injected into the mouse brain and treated with an experimental therapy.
These studies have identified many candidate approaches; mice with glioblastoma are often “cured”, but these studies rarely translate into effective therapies in humans. The reasons for this Bench to Bedside barrier are many. Mouse models are good at identifying toxicities but cannot model the true glioblastoma complexity. Pre-clinical studies often use responsive cells with higher doses of drugs that may be applicable in patients, and treatment is often started within a few days of tumour implantation, lowering the bar for success enormously.
Moreover, the scale of the mouse brain is much different to that of a human and often, these models lack key hallmarks like invasiveness, heterogeneity and immunosuppression to the extent seen in human glioblastoma. Improved models, both in animals, and ex vivo organoids derived from human cells, would provide a higher bar for therapeutic efficacy, and help overcome this critical bench to bedside barrier. With urgency, teamwork, funding and support, we hope that the remarkable efforts of scientists and clinicians will soon provide the improved outcomes that brain cancer patients and their families so dearly wish for.
Please note: This is a commercial profile