Transcending morphology, magnetic resonance techniques can be utilised to shed light on processes on the molecular level to unveil pathological alterations preceding anatomical and functional manifestations of (cardiovascular) diseases, in the view of Ulrich Flögel from Heinrich Heine University Düsseldorf, Germany
Today, magnetic resonance imaging (MRI) is undoubtedly the leading technique in diagnostic imaging. It has attracted a great deal of interest because of its unique combination of qualities. MRI uses non-ionizing radiation, which is harmless to human tissue, offers very high image quality with excellent anatomical details and additionally is capable of providing not only functional but also metabolic information. On the negative side, it was conventionally argued that MRI suffers from low sensitivity compared to other imaging modalities. However, a new generation of nanotechnology-based contrast agents is making it possible to overcome this limitation and bring MRI into the molecular imaging category.
In this context, fluorine (19F) MRI has lately garnered significant scientific interest in the biomedical research community, due to the unique properties of fluorinated materials and the 19F nucleus. The stable fluorine isotope 19F naturally occurs to 100% and exhibits an intrinsic sensitivity for MRI close to that of the 1H nucleus, which is commonly used to produce detailed images of the inside of the body as has been addressed recently. There is negligible endogenous 19F in the body and, thus, no background signal which allows the detection of fluorinated materials as ‘hotspots’ by combined 1H/19F MRI and renders fluorine-containing molecules as ideal tracers for a wide variety of MRI applications. Importantly, there is a family of compounds – perfluorocarbons – which exhibits a very high fluorine payload and is biochemically and physiologically inert (e.g. Teflon).
However, perfluorocarbons are not mixible with water, therefore they have to be dispersed in water by use of an emulsifying agent leading to biologically applicable perfluorocarbon nanoemulsions (PFCs). Of note, some of those emulsions containing perfluorodecalin, perfluorotripropylamine, perfluorodichloroctane and perfluorooctyl bromide (also known as perflubron or Oxygent®) were already used in patients as artificial blood substitutes.
The recent application of those PFCs for molecular imaging takes advantage of the fact, that after intravenous administration an efficient and selective uptake of PFCs by circulating cells of the innate immune system, in particular monocytes and macrophages, takes place. The subsequent migration of the 19F-loaded, immunocompetent cells into inflammatory foci then permits the unambiguous in vivo identification of affected regions by combination of 1H and 19F MRI. This is illustrated in a murine model of myocardial infarction induced by occlusion of a coronary artery – a procedure well known to be associated with an acute inflammatory response in the affected tissue.
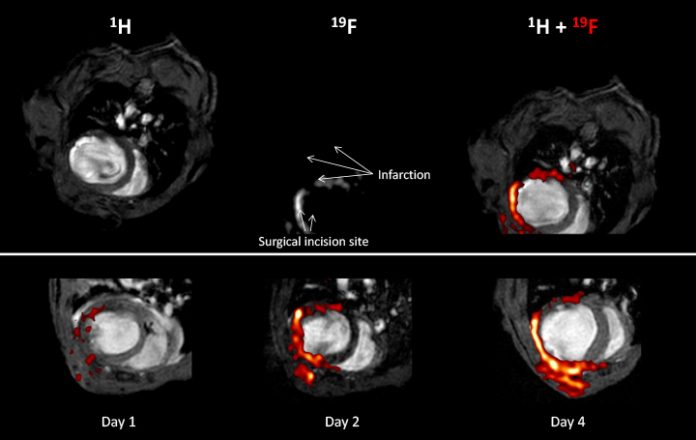
The upper row demonstrates the principle of the combined 1H/19F MRI approach: The end-diastolic 1H image (left) clearly shows the presence of ventricular dilatation and wall thinning within the infarcted area of the heart and in the corresponding 19F image (middle), the signal pattern matches the shape of the free left ventricular wall. For merging of the original grayscale images, a hot iron lookup table is applied to the fluorine data, which allows convenient discrimination of the signals from the 1H and 19F nucleus. The resulting overlay (right) confirms the localisation of PFCs within the anterior, lateral and posterior walls. Furthermore, 19F signal is also detected in the adjacent chest tissue, where thoracotomy for the surgical intervention was performed. Note that otherwise no background 19F signal from other tissue is present.
Repetitive measurements from day one after ligation of the coronary artery reveal a time-dependent accumulation of PFCs within the infarcted region as shown in the lower row. The end-diastolic 1H images acquired one, two and four days after induction of myocardial infarction show the progressive left ventricular dilatation as a consequence of the insult. Merging with the matching 19F images (red) demonstrates the successive infiltration of PFCs into the affected area of the heart and the region of the chest injured by surgery. Detected 19F signals are restricted to the area near the infarcted region of the heart; at no time infiltrating PFCs are observed within the unimpaired septum.
This suggests that not only is it possible to clearly detect specific areas of inflammation, but it is also feasible to assess the severity of inflammation damage. Thereby, the described approach offers a wide range of therapeutic potential: a better understanding of the degree of inflammation in multiple disease conditions would hugely benefit individuals, providing an opportunity to offer timely patient-specific therapy and the capability to monitor treatment regimes.
Forging the way ahead, active targeting of PFCs – by decoration of the particle surface with ligands binding to specific epitopes – can be exploited to visualise other clinically relevant structures including blood clots and different cell types. By attaching distinct ligands for different targets to individual PFCs that each has its own specific MRI signature, this will allow the concurrent visualisation of several danger signals within one MR session. Here, the ultimate goal will be to advance the 19F probes for in vivo visualisation of biomolecular processes, which proceed alterations at the anatomical and organ level and to enable early patient-specific precision therapy.
Finally, in terms of a theranostic approach, targeted PFCs could additionally be doped with distinct drugs, which will enable, by directing those PFCs to a specific danger pattern, its concurrent treatment. Since ligands and targets can be easily adapted to a variety of problems, this approach provides a general and versatile platform for molecular imaging which strongly extends the frontiers of MRI. Thus, in the near future, this exciting emerging field of 19F MRI has the potential to revolutionise imaging-based cell tracking, imaging of disease and its subsequent therapy.
Please note: This is a commercial profile