Professor of Evolutionary Biology Ashleigh S. Griffin discusses harnessing the power of evolutionary process instead of fighting it, particularly regarding bacterial cells and cooperation
When I was growing up, nobody had heard of meerkats. But in 1986, the BBC showed a documentary called “Meerkats United” and their popularity has continued to grow ever since. The reason they captured the public imagination so vividly is because of their incredible sociability – their cooperation in rearing one another’s pups, coordinated attacks against intruders and predators, and complex systems of communication, rarely seen in other creatures besides us.
Social bacteria
It might surprise you to learn that bacterial cells are far more cooperative than meerkats, or human beings, or any other animal: Self-sacrifice is central to their strategy to survive in almost every environment on the planet, including inside our bodies when they are causing infection.
Cooperation is defined by evolutionary biologists as an action that benefits another individual, and that has evolved at least partially because of that benefit. Organisms do not need a brain or intention to perform behaviours under this definition. Daily functions crucial to a bacterial cell’s survival rely on their ability to cooperate with one another. For example, let’s take feeding. We feed by ingesting food items and processing them in our gut. We produce enzymes to break proteins into amino acids, or carbohydrates into glucose, which we can then absorb through our gut membrane.
Bacterial cells don’t have a gut. They must digest their food outside of their bodies and then hope to absorb amino acids and sugars back through their cellular membranes where they can be used for metabolism. Bacteria typically live in densely packed, clonal communities and so, this process is almost impossible to perform without helping your neighbours.
Cooperation is key
In economic terms, digestive compounds produced by bacteria are a common-pool resource, like fish in the North Sea. The supply is finite but available to all. And just like fish in the sea, bacterial common pool resources are vulnerable to exploitation. Over the last 20 years, my research, along with that of others, has revealed the complex social interactions that take place in bacterial communities. More recently, we have shown that social interactions between bacterial cells can account for long-term evolutionary changes observed in the lungs of patients with cystic fibrosis. But here I want to explain what all this has to do with the fight against antibiotic resistant bacterial infection.
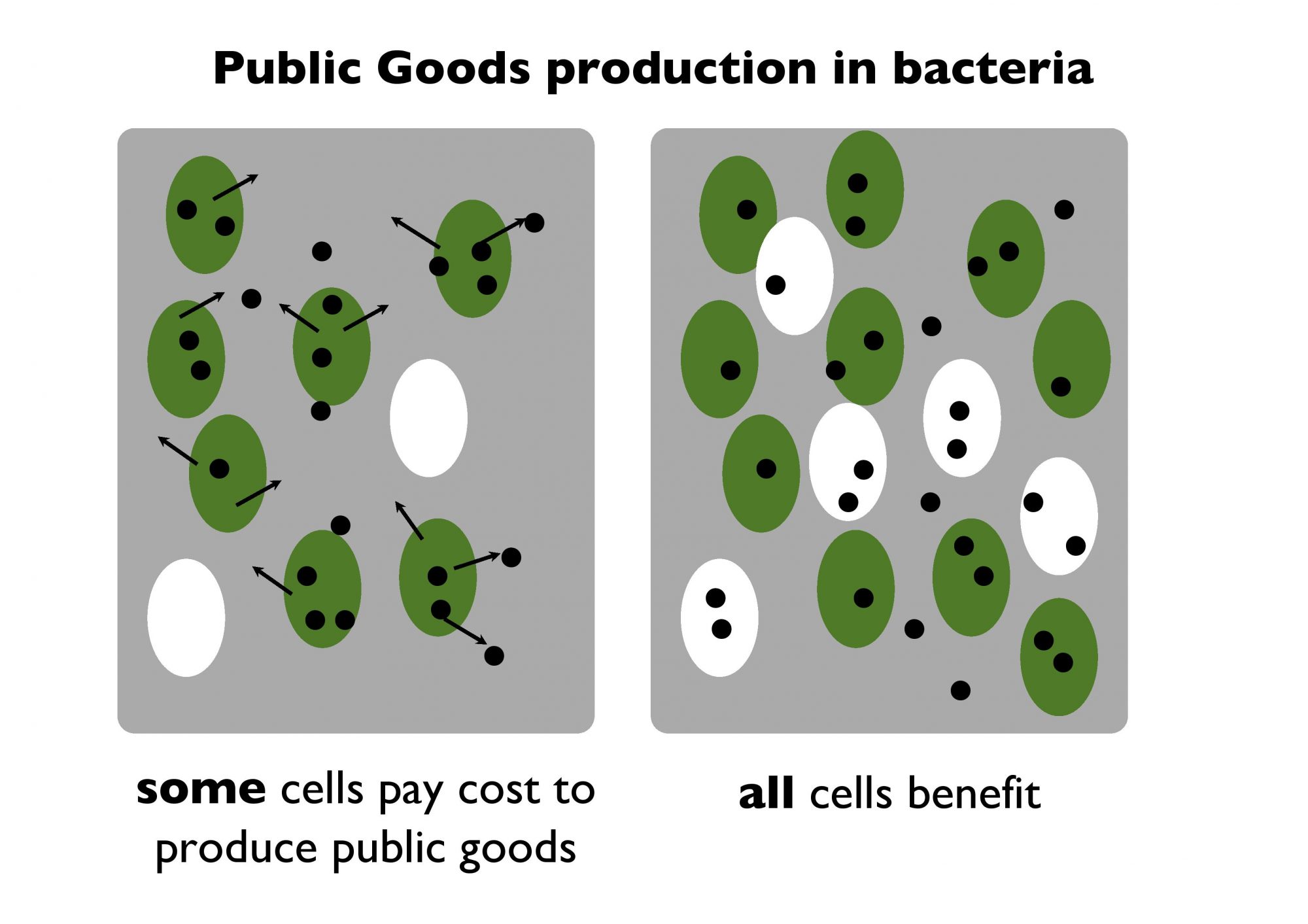
Bacteria have evolved different strategies of resistance against antibiotics. Some of these strategies are social, some are not. Bacteria have evolved “pumps” to expel antibiotic compounds which manage to get inside the cell. This only benefits the pumping cell: Antibiotics are expelled completely intact and unharmed back out into their environment where they may kill neighbouring bacterial cells. Other mechanisms involve the release of enzymes called beta-lactamases into the environment, where they can degrade antibiotic compounds before they have a chance to enter cells and cause damage.
This is cooperative resistance: Beta-lactamase is a common-pool resource which benefits neighbours as well as the cell that produces them by reducing the concentration of antibiotic in the shared environment.
Treating with cheats?
The exciting prospect we are currently working on, with funding from the European Research Council, is being able to manipulate cooperative interactions between bacterial cells for clinical benefits. The potential strategies we are exploring rely on the old adage “cheats prosper.” We can exploit evolutionary processes that promote the spread of social cheats to lower the virulence of infections and even completely alter the genetic composition of an infection to make it more vulnerable to antibiotics.
Even if that resource is crucial for survival, it may have the chance to thrive by exploiting its more cooperative neighbours. Remember that our mutant is likely surrounded by neighbours pumping out enzymes that it can benefit from, without paying the cost of contributing. It may have higher fitness and be able to spread, even if that means the population as a whole going extinct. Although this is bad news for the bacteria, it can be good news for us. Cheats that no longer make these compounds are less effective pathogens. Starving bacteria that lack a crucial enzyme for growth may die, or grow more slowly. We have shown experimentally that “cheating” bacteria are less virulent and can invade wildtype cooperative bacterial communities.
Could we then apply live bacterial culture to treat infection? It might sound paradoxical, but we already use live bacteria in treatments, for example probiotics in the treatment of upset stomachs.
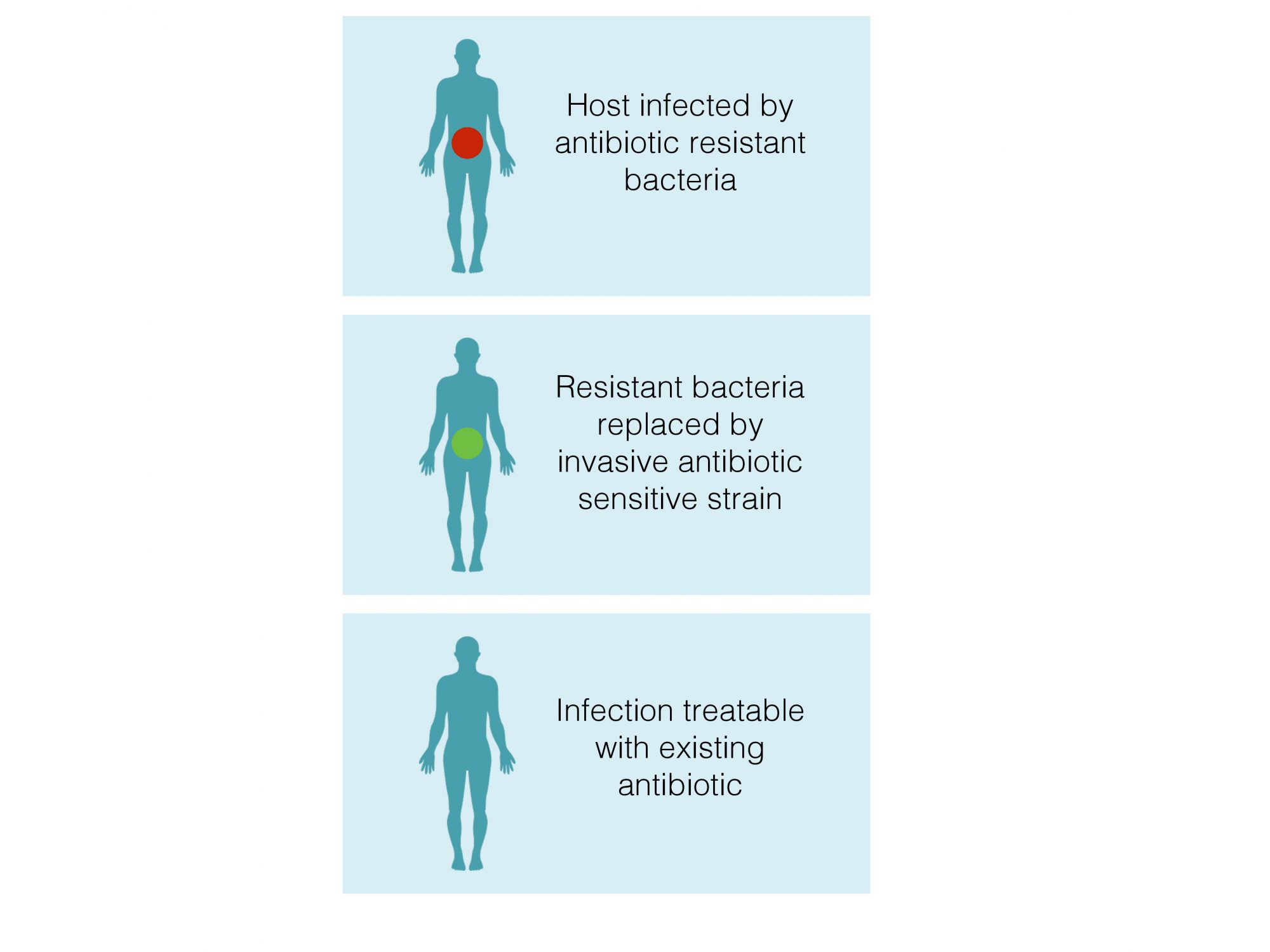
The second strategy we are exploring takes this idea one step further: exploiting the invasive ability of cheats to drive medically beneficial genes into infection. We can genetically engineer social cheats to become sensitive to antibiotics, for example. When cheating cells invade by exploiting cooperative bacterial populations, they will take the antibiotic sensitivity with them. We think of this as a Trojan Horse strategy where the cheat is the horse and the gene we want to introduce are Greeks who wouldn’t have otherwise been able to overcome the defences of their target population.
What the future holds
The dream is that by harnessing the power of evolutionary process instead of fighting it, we could restore sensitivity to old antibiotics that we currently can’t use any more, or “de-fang” bacterial infections enough to give existing drugs or our natural immunity a chance to work. There are many challenges facing attempts to make this work.
But with the average shelf life of a new antibiotic being two to three years, making antibiotic drug discovery economically inviable, we need to start thinking differently about how to deal with bacteria. Rather than trying to eradicate them cell-by-cell, we should maybe start to think about how we can mess with their social lives.

Social evolution and Social engineering (SESE) has received funding from the European Union’s HORIZON 2020 Research programme under the Grant Agreement no. 647586.
*This is a commercial profile.
© 2019. This work is licensed under CC-BY-NC-ND.











