Associate Professor Petr Broz discusses pyroptotic cell death, the controlled cellular explosion that can harm your health
Programmed cell death is an essential biological process that is necessary for embryonic development, tissue homeostasis as well as the removal of cancerous or infected cells. The most common cell death pathway is called apoptosis and constitutes a tightly regulated program that results in cell shrinkage and the formation of apoptotic bodies, which are engulfed and digested by phagocytic cells, like macrophages. Importantly, apoptosis is an immunologically silent process that does not cause any inflammation.
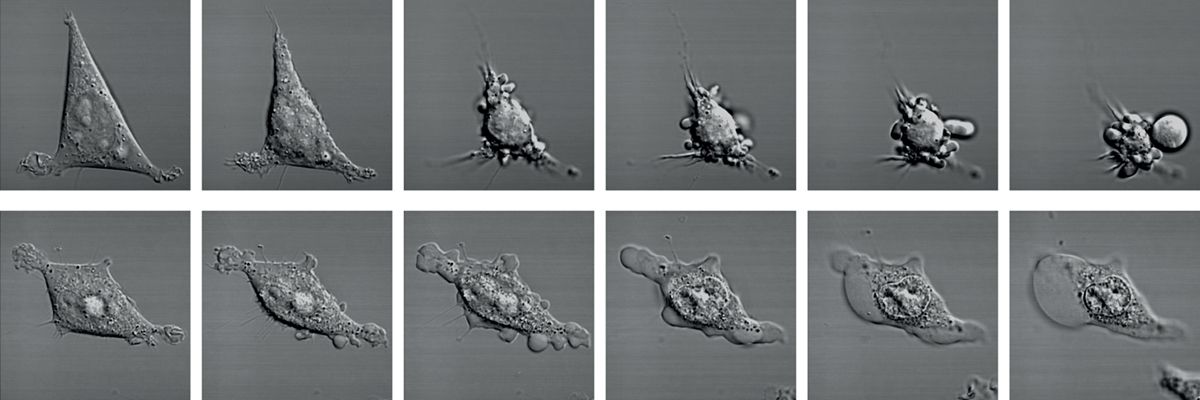
Almost 20 years ago a new form of lytic cell death – called pyroptosis – was identified that contrasts starkly with apoptotic cell death. During pyroptosis, cells enlarge and balloons within minutes, until it explodes. This cell explosion was initially thought to be accidental, as it was first observed in cells infected with pathogenic bacteria or viruses. However later work showed that pyroptosis is part of a well-orchestrated response to sterile and infectious triggers, that is coordinated by central signalling hubs of the human immune system, the inflammasome complexes.
Inflammasomes
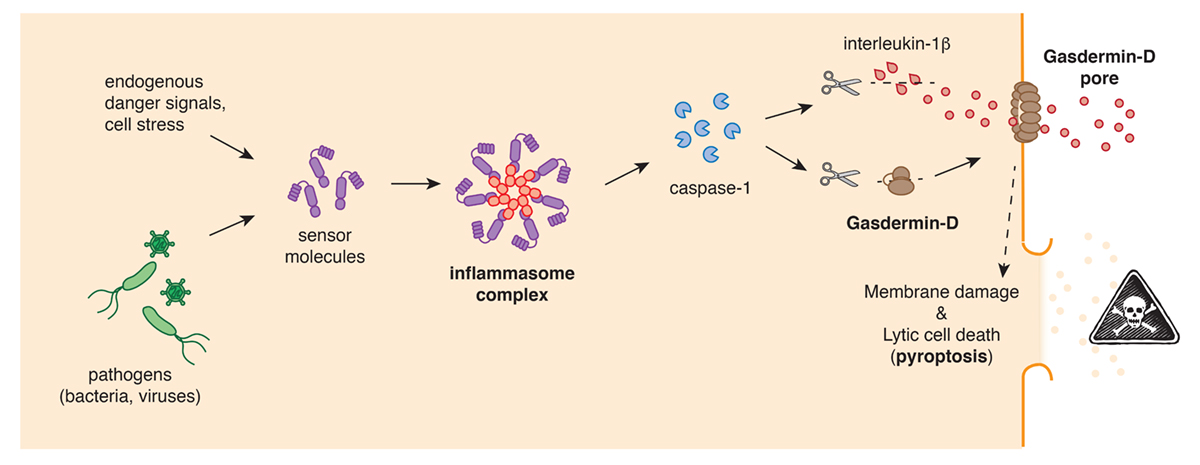
Inflammasomes, are multi-protein complexes that are assembled by cellular sensor proteins that surveilled the cytosol of cells and respond to danger such as infections, cancer or toxic molecules. Once an inflammasome complex is formed, it will rapidly recruit and activate caspase-1, a protease that is essential for the induction of pyroptotic cell death and the subsequent release releases of cellular content and highly-proinflammatory cytokines, like interleukin-1b (IL-1b), that cause fever.
The best-known member of these inflammasome-forming sensors is NLRP3, which is also known as Cryopyrin and which in recent years has garnered attention from well over a dozen drug companies due to its importance in many human diseases. Mutations in NLRP3 are known to cause a number of rare genetic disorders, collectively referred to as cryopyrin-associated periodic syndromes (CAPS), that are characterised by uncontrolled inflammation and fever. In addition to this, the NLRP3 inflammasome has been shown to contribute to the inflammation that underlies diseases like gout, atherosclerosis, nonalcoholic steatohepatitis (NASH) and type II diabetes, but also neurodegenerative disorder like Parkinson’s and Alzheimer’s disease.
Patients with CAPS are currently prescribed IL-1β inhibitors or IL-1 receptor antagonists, like Anakinra, Rilonacept or Canakinumab. But targeting NLRP3 activation directly could proof to be a more specific means to reduce inflammation. The most-advanced compound here is a molecule dubbed MCC950, that functions as a selective NLRP3 inhibitor. MCC950 has by now been tested in up to 80 animal models of more than 50 diseases and is able to specifically inhibit NLRP3-driven inflammation in vivo.
Gasdermin-D, a pore-forming cell death executor
But how do inflammasomes induce pyroptotic cell death? The answer to that question emerged in 2015, when two research groups simultaneously identified gasdermin-D as the central gatekeeper of pyroptosis. The Gasdermin-D protein, which is normally inactive and non-toxic, was also shown to be cleaved by the protease caspase-1 into two parts. It is this cleavage that removes an inhibitory part and thereby converts the rest of the protein into a highly effective cell killer. The activated gasdermin-D seeks out cellular membranes, where integrates and forms large pores. The permeabilisation of the cellular plasma membrane by gasdermin-D pores allows inflammatory molecules and the cytokine IL-1b to spill out of the cell, and eventually, the pores cause the cells to completely burst.
As the gatekeeper of the inflammasome pathway, gasdermin-D is an attractive target for pharmacological inhibition of pyroptosis and the associated cytokine release. Indeed, animal studies have shown the genetic deletion of gasdermin-D prevents the inflammation in mouse models of sepsis, CAPS and familial Mediterranean fever, an autoinflammatory disease caused by mutations in the inflammasome-sensor Pyrin. Furthermore, first studies have shown that small-molecule inhibitors of gasdermin-D are able to prevent sepsis-associated lethality in mouse models, but there is still a long way to go until the development of specific gasdermin-D inhibitors.
Gasdermins- beyond the inflammasome
What makes gasdermin-D even more important, is that this protein is only one in a larger family of similar proteins, that all share the ability to form pores and induce pyroptotic cell death. So far, a total of 6 gasdermins were identified in humans and recent research has begun to uncover their role in human health and disease.
The best-studied of these gasdermin is Gasdermin-E, a potent tumour suppressor gene, but whose expression is often mutated or silenced in many tumours. Recently it could be shown that reactivation of Gasdermin-E expression within tumour cells causes a rerouting of tumour cell death during cancer chemotherapy from apoptosis towards pyroptosis. Since pyroptosis is highly inflammatory, this forms an inflammatory environment around the tumour that attracts many kinds of anti-tumour immune cells and makes the tumour recognisable to the immune system as a threat.
Uncontrolled gasdermin activation, however, can also prove to be a problem for cancer therapy. Chemotherapy drugs for example, cause excess inflammation by causing pyroptosis through gasdermin-E. Furthermore, gasdermin-E-driven tumour cell pyroptosis has also been proven to be the cause of cytokine release syndrome, that causes low blood pressure, fever, nausea, and breathing problems in patients, and that is a common side-effect of Chimeric antigen receptor (CAR) T cell therapy. Here pyroptotic tumours cells release cellular content that the NLRP3 inflammasome in tissue macrophages and the production of IL-1b. Interfering with gasdermin activation or the NLRP3 inflammasome could thus prove to be a key step in making CAR T cell therapy safer.
Final remarks
Rapid progress in the last 20 years has led from the first observations of pyroptosis in tissue culture models to a molecular understanding of its biology and its consequences. However, only now the biological significance of pyroptosis has begun to emerge both as a pathological process during autoinflammatory diseases, or as a desired outcome of the host response to infections and cancer. Because of this pivotal role, manipulating pyroptosis itself or the signalling pathways underlying its activation holds a great promise for the development of new therapies for both inflammatory diseases and cancer.
Please note: This is a commercial profile