Drs Alfred Msezane and Kelvin Suggs discuss the possibilities of efficient tunable water oxidation to peroxide catalyzed by doubly charged atomic negative ions
The fundamental mechanism underlying negative-ion catalysis involves hydrogen bond strength-weakening/breaking in the transition state. Doubly charged atomic negative ions are proposed for dynamic tunable catalysis of water oxidation to peroxide for a protective environment. Density Functional Theory transition state calculations have found Pu-2, Sn-2 and La-2 the best catalysts among the investigated anions in reducing the energy barrier considerably. Our results indicate tunable barrier activation energies from 0.087eV to 0.884eV; the potential applications range from water purification to biocompatible anti-viral and anti-bacterial sanitation systems.
Introduction
Recently, the Royal Society of Chemistry published the themed collection “Single Atoms as Catalysts” [1] to celebrate the International Year of the Periodic Table. This has been followed by the investigation of single fullerene negative ions as catalysts [2]. Their effectiveness has been demonstrated in catalysing the oxidation of water to peroxide and water synthesis from H2 and O2 using the fullerene anions from C44ˉ to C136ˉ. Negative ion catalysis involves anionic molecular complex formation in the transition state, with the atomic negative ion weakening/breaking the hydrogen bond strength. In [2] Density Functional Theory (DFT) found the C60ˉ anion to be optimal; namely, reduce the transition state (TS) energy barrier by the same amount in the oxidation of water to peroxide and water synthesis from H2 and O2. Importantly, DFT also found the C136ˉ anion to represent the best catalyst, among the investigated anions, for both water oxidation to peroxide and water synthesis from H2 and O2.
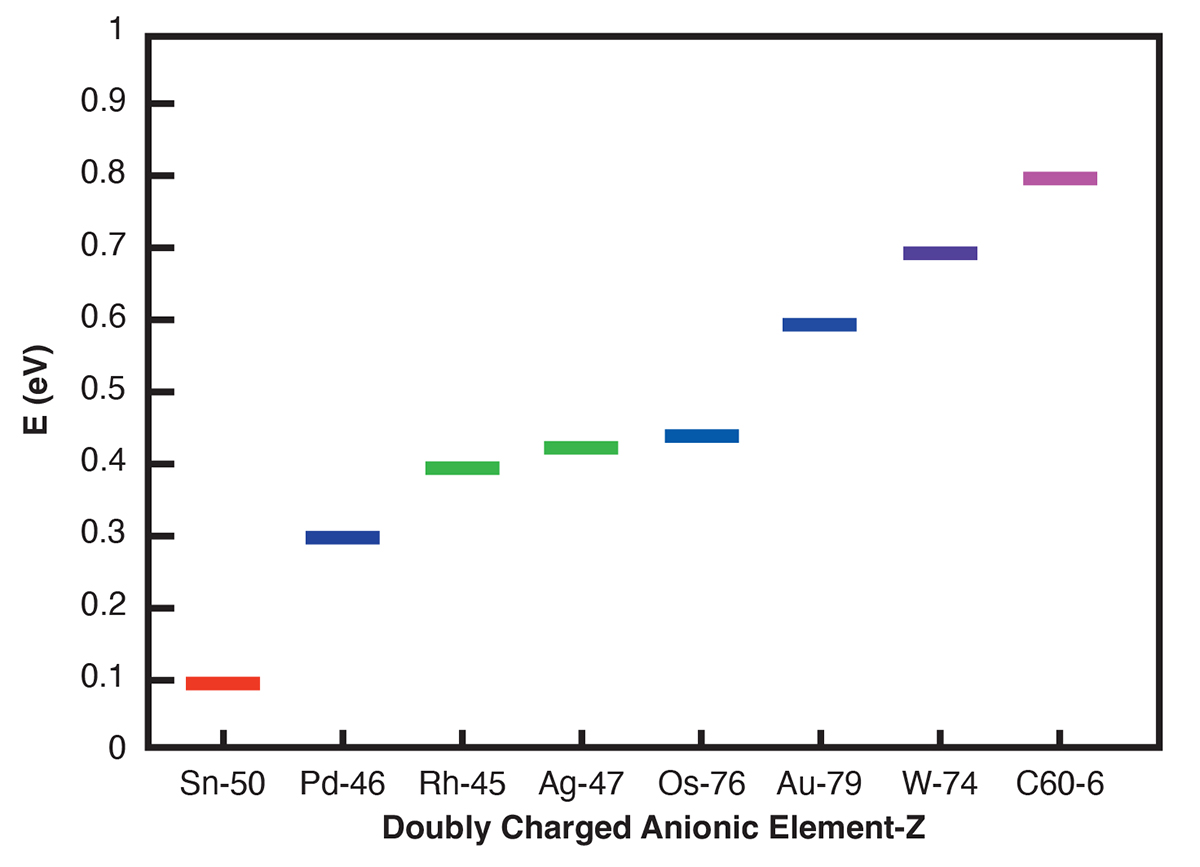
Here we have selected the doubly charged standard reference C60ˉ2 anion, the negative metal ions Sn-2, Pd-2, Ag-2 and Au-2, the lanthanide ions La-2, Ce-2, Eu-2 and Tm-2 and the actinide anions Th-2, Pa-2, Pu-2 and U-2 to investigate their effectiveness in catalysing the oxidation of water to peroxide. The selection of the Sn-2, Pd-2 and Au-2 anions has been motivated by the successful experiments of Hutchings and collaborators [3] in catalysing H2O2 from H2 and O2 using Au, Pd and Sn nanoparticles. In particular, the experimenters [3] concluded that the inexpensive atomic Sn could be useful for water purification in the developing countries. Density Functional Theory and Regge pole analyses have been used in the selection of the investigated doubly-charged negative ions. DFT transition state calculations concluded that the Pu-2, Pa-2, Sn-2 and the La-2 catalysts reduce the energy barrier the most, followed by Eu-2 when compared with the rest of the doubly charged atomic systems.
The standard benchmark reference C60ˉ2 TS value has been found to be 0.784eV. This value should be compared with the 1.94eV obtained for the C60ˉ [2]. This implies that further reduction of the charge of C60ˉ by -1 reduces the energy barrier by a factor of about 2.5 (1.94eV versus 0.784eV).
Additionally, our results indicate doubly charged anionic tunable barrier activation energies varying from 0.087eV to 0.884eV for the investigated atoms with potential applications ranging from water purification to biocompatible anti-viral and anti-bacterial sanitation systems.
Results
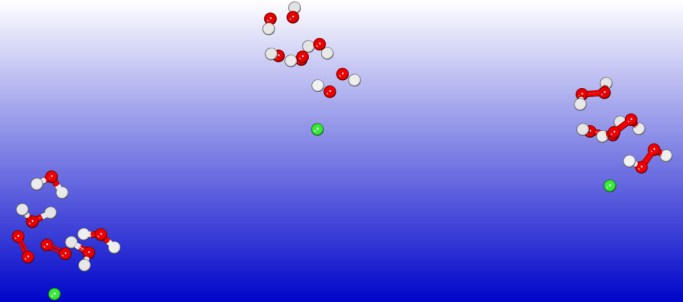
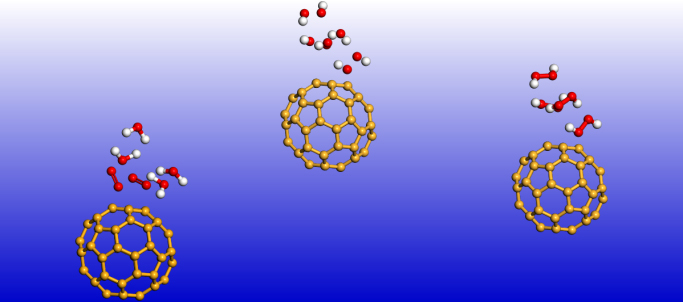
For the TS calculations DFT and dispersion corrected DFT approaches have been employed. The gradient corrected Perdew-Burke-Ernzerhof parameterisations [4] of the exchange- correlation as implemented in DMol3 [5] was used for the geometry optimisation of the structural molecular conformation. A tolerance of 1×10-2Ha was used with a smearing value of 0.1Ha. We demonstrate in Figs. 1 and 2 the doubly charged anionic TS calculations of Sn and C60 when catalysing water to peroxide. The popular C60ˉ2 anion possesses a barrier of 0.784eV. However, Pu-2 exhibits the most efficient barrier of 0.087eV(Fig. not shown), followed by Sn-2 with 0.103eV. The results of the various metal elements are summarised in Fig. 3.
Concluding Remarks
Previously, cationic systems were discovered to increase the TS energy barriers in the synthesis of peroxide from H2O[6]. This could render them essential as inhibitors in controlling and regulating catalysis. Conversely, doubly charged anionic systems may prove to be the effective reaction promoters. These calculations provide further insight into the fundamental and comprehensive atomic theoretical framework for the next generation of tunable catalytic systems.
We conclude that the anionic catalysis of the oxidation of water to peroxide is further optimised and thereby tunable via purely electronic means with Sn-2 being the best candidate as validated by the experiments [3]. The results may also be applicable to the petrochemical waste reduction of SO2 by CO as demonstrated in[7] as well as in the conversion of methane to methanol without CO2 emission. The doubly charged lanthanide (La, Eu) and actinide (Pu, Pa) anions are also competitive catalysts in the oxidation of water to peroxide. The complete results of this investigation which will also include the interesting and revealing results of the lanthanide and actinide atomic anions will be presented in our forthcoming publication on the subject of catalysis by doubly-charged atomic metal, graphene, and fullerene negative ions.
Acknowledgments: Research was supported by the U.S. DOE, Grant: DE-FG02-97ER14743. The computing facilities of National Energy Research Scientific Computing Center, also funded by U.S. DOE are greatly appreciated.
References
1. International Year of the Periodic Table: Single Atoms as Active Catalysts. Available online: https://pubs.rsc.org/en/journals/articlecollectionlanding?sercode=nr&themeid=1fc90a67-e081-4265-99eb-2201eb17c286 (accessed on 12 January 2017).
2. Felfli, Z.; Suggs, K.; Nicholas, N.; Msezane, A.Z. Fullerene Negative Ions: Formation and Catalysis. Int. J. Mol. Sci. 2020, 21, 3159.
3. Freakley, S.J.; He, Q.; Harrhy, J.H.; et al. Palladium-tin catalysts for the direct synthesis of H2O2 with high selectivity. Science 2016, 351, 959.
4. Tkatchenko, A.; Scheffler, M. Phys. Rev. Lett. 2009, 102, 73005
5. DMol3 2011 Accelrys Software Inc.: San Diego, CA
6. Tesfamichael, A.; et al. JNR 2013, 15 1333
7. Hu, Y.-L.; et al. Chin. Phys. B 2019, 20, 113101
*Please note: This is a commercial profile