Here, we learn how world leading supplier Bioquell devised a PRP for a Biopharmaceutical Production Facility ensuring high-level bio-decontamination in the event of contamination
A multinational biopharmaceutical company in Asia, was looking to introduce a rigorous plan to ensure high-level bio-decontamination of their manufacturing facility in the event of contamination.
The customer chose Bioquell’s Rapid Bio Decontamination Service (RBDS) to produce a Priority Response Plan (PRP) that enables swift mobilisation of RBDS personnel and equipment. It also reduces the risk of unforeseen challenges thus minimising downtime of the manufacturing facility. Bioquell RBDS performs hydrogen peroxide vapour decontamination that achieves a rapid 6-log sporicidal reduction on all exposed areas within an enclosed environment. The process is scalable to cover various needs should a contamination event occur.
Every deployment is fully documented with detailed results verified using biological and chemical indicators, ensuring a successful decontamination, as well as covering any regulatory and auditing concerns.
The PRP reduces the risk of unforeseen challenges and provides a guaranteed rapid response to contamination events.
Background, requirements, and set-up
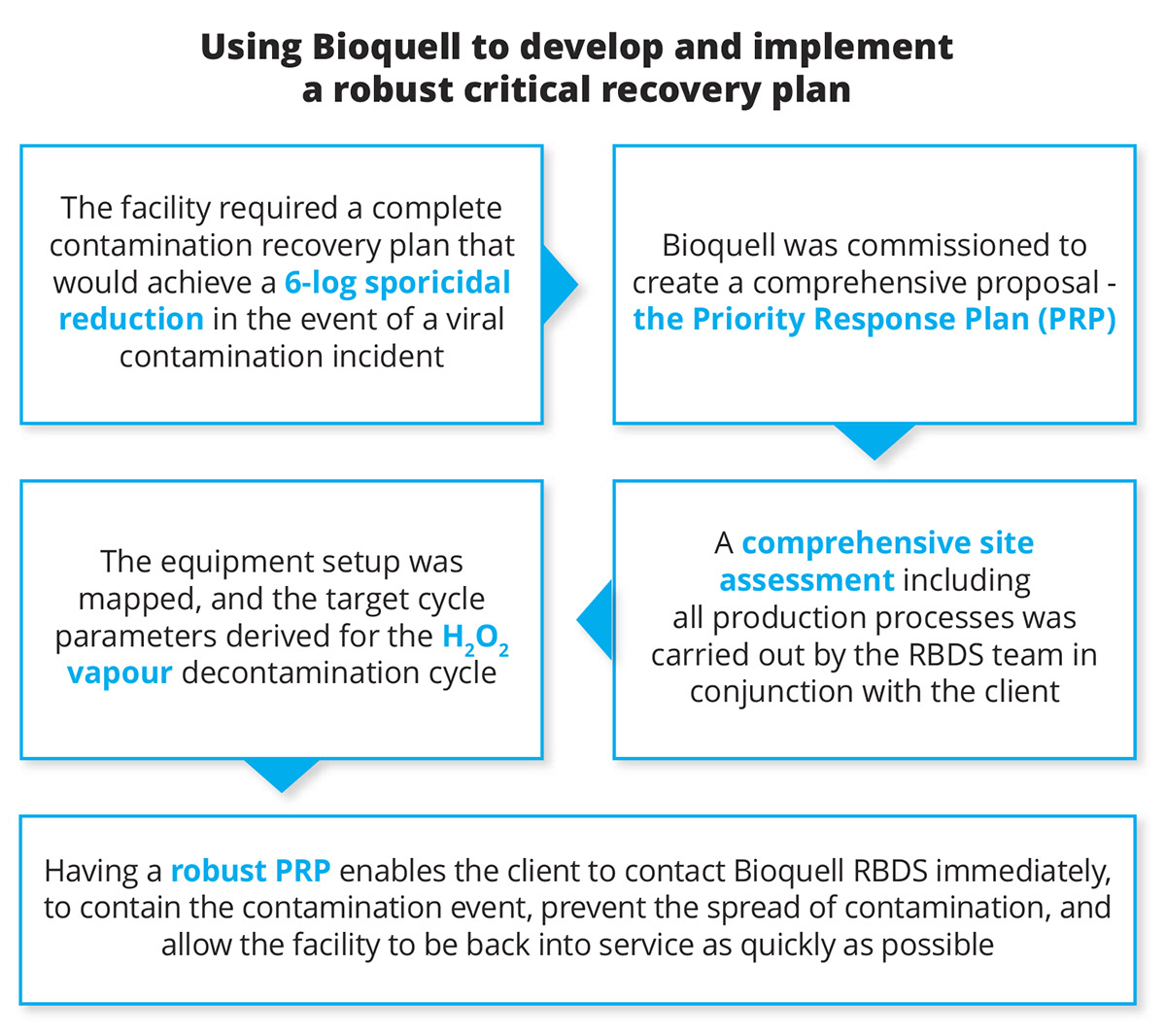
A leading multinational biopharmaceutical company in Singapore required a complete contamination recovery plan that would achieve a 6-log sporicidal reduction in the event of a contamination incident within its state-of-the-art biomanufacturing facility. Although there was no active contamination incident to deal with, the company understood the importance and impact that a bio-decontamination recovery plan could provide as a precautionary measure should a viral contamination event be discovered.
Challenge
Efficacy: A full PRP was required to enable an effective decontamination process which could be validated by the client to remove any potential bioburden within the manufacturing areas being targeted.
Documentation: The PRP documentation is a vital part of the client’s business continuity planning, giving them a robust list of procedures should a contamination emergency arise. In the event of a contamination incident, the Bioquell RBDS process is fully documented for auditing and regulatory inspections.
Solution
The biopharmaceutical company was impressed by Bioquell’s expertise in writing PRP plans for clients in Europe and the United States and, after reviewing the other bio-decontamination options in the market, Bioquell was commissioned to create the comprehensive PRP.

For the client, having a PRP in place offers a number of additional benefits over and above using the standard RBDS service in the event of a contamination incident. Procedures have already been mapped, increasing the speed to mobilise, and reducing the risk of unforeseen challenges for both the client and the Bioquell RBDS team. The facility has already been surveyed, allowing swift deployment of Bioquell RBDS equipment to pre-defined locations dependent on where the contamination event has occurred. A rapid response in this instance is made possible due to Bioquell’s presence in Asia along with a dedicated, local RBDS team and equipment.
In the event of a decontamination incident, the hydrogen peroxide vapour technology offered by Bioquell’s RBDS generates a 6-log sporicidal kill on all exposed surfaces and is validated against Geobacillus stearothermophilus Biological Indicators (BIs), considered the industry standard validation benchmark for determining hydrogen peroxide vapour decontamination efficacy.
Outcomes
Approach: The Global RBDS Manager and the client’s Account Manager carried out a full and thorough site visit. This included a review of the full production process including the raw material flow, the HVAC systems, pressure regimes and cleaning and gowning protocols. A detailed discussion with all pertinent stakeholders was critical to ensure all practical aspects of performing an emergency bio-decontamination were adequately covered. Conducting this site survey was vital to enable the Bioquell RBDS team to map the equipment setup and derive the target cycle parameters for the hydrogen peroxide vapour decontamination cycle.
The PRP was created on a zone by zone basis based on the survey of the facility. The plan had to be modular to allow one or more zones to be treated at once if required. After the site survey was completed, Bioquell RBDS prepared the PRP document and presented it for approval.
“Having a Priority Response Plan (PRP) in place offers a number of additional benefits over and above using the RBDS service.”
Results: Bioquell RBDS produced a comprehensive PRP document that establishes a robust process to allow a rapid bio-decontamination deployment in the event of contamination at the client’s facilities.
The plan enables trained Bioquell RBDS personnel to read the file and perform a complete decontamination process that will achieve a 6-log reduction in bioburden in one area or the whole of the biomanufacturing facility. The PRP also enables the company’s personnel, who may have no prior knowledge of a decontamination process, to be able to prepare the areas for decontamination.
A Programme to Decontaminate up to 4200m3.
A 6-log sporicidal reduction can be achieved with Bioquell RBDS equipment.
A PRP providing rapid bio decontamination is now in place.
Use Bioquell products safely. Always read the label and product information before use.
*Please note: This is a commercial profile
Ecolab
Tel: 1 215 682 0225