Here, we learn how international diagnostics group Novacyt are leading the innovation revolution in clinical diagnostics through discovery, design, and innovation
An innovation revolution is underway in clinical diagnostics, fuelled by the rapidly evolving demands of the COVID-19 pandemic and highlighted by the high unmet medical need for rapid, near-patient diagnosis.
That is the view of Trevor (Ajan) Reginald, Chief Technology Officer at Novacyt, who sees the company in pole position to build on its pandemic track record for the post-COVID-19 environment.
“We are now in a unique time in which, most people in the UK expect to be able to take a clinical diagnostic test in their local town and receive the result in a few days. And even in less-resourced countries, the work of the UNICEF and NGOs has created a far better global diagnostics footprint,” adds Ajan.
Agile response
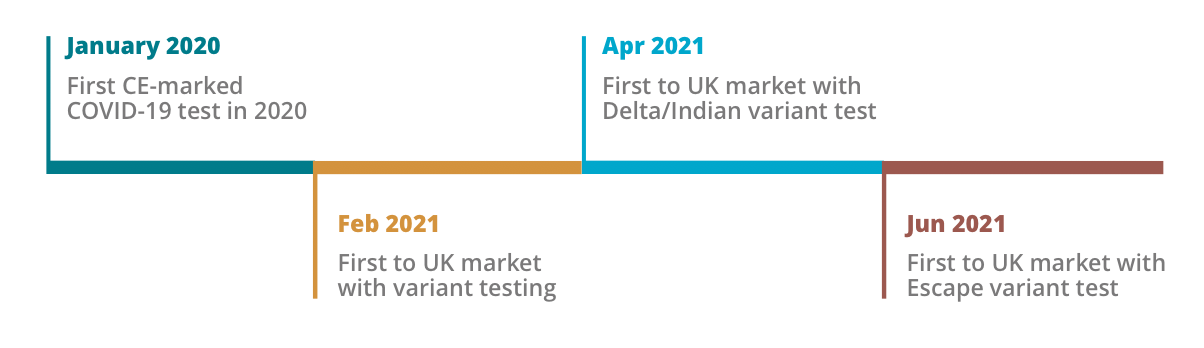
Novacyt has a heritage of responding first to potential pandemics by rapidly developing assays for Bird (H1N1) flu (2009), Ebola (2014), and Zika (2016) with first to market breakthrough diagnostics tests. Their response to the SARS-CoV-2 outbreak proved no exception.
“Novacyt excels in the discovery, design and innovation of new medical diagnostics,” explains Ajan. “We have robust and detailed analysis, and our automated platform analysing more than two million genetic sequences per week to identify mutations – a huge scale up from the 10,000 per week pre-pandemic. And through detailed, diligent analysis, our design team is able to create best-in-class assays designs with ~99% sensitivity and specificity.”
“The pandemic has accelerated the innovation and implementation of medical diagnostics. Awareness, access and testing infrastructure has rapidly expanded albeit with perhaps, an exacerbation of existing health inequalities. Prior to COVID, the perception and awareness of diagnostics lagged Biotech and Pharma but the pandemic put the industry on war footing, and we’ve seen significant advances in innovation to generate a class of exquisite diagnostics. This provides perhaps a once in a generation opportunity to build a diagnostics industry (akin to Biotech in the 90s), that can significantly advance the human healthcare.”
The pandemic fuelled exponential growth in new diagnostic development. We launched two new products in 2019 to 25 in 2021. Novacyt’s innovation is its core strength, with a track record of rapid agile development:
Clinical evidence
Novacyt’s growing ability to run clinical trials, and its in-house regulatory expertise, have been lynchpins of the company’s pandemic response.
Our integrated approach combines regulatory, discovery and development teams. “From discovery, development, clinical trial design, we have a fully integrated iterative loop which is highly innovation-focused and patient-centric,” says Ajan. “We develop tests that are faster, better or easier to use – ideally all three – so that clinicians are able to make the highly accurate time-sensitive diagnosis needed to improve patient outcomes. This is a recurring area of unmet medical need in which focus our innovation. We can demonstrate this clinical benefit through well designed clinical trials and because we’re addressing high unmet needs, clinicians are very keen to be involved. Clinical trials showcase the best-in-class clinical performance of our assays within a clinical use case e.g., Emergency rooms, which then facilitates the understanding and uptake of new innovation by clinicians.”
As regulators increasingly demand high-quality clinical evidence for medical diagnostics, clinical trials are essential to generate high-quality patient data for regulatory filings and market access. Since Q4 2020, Novacyt has run 15 trials in more than 20 UK and international markets.
Manufacturing flexibility
Novacyt was also able to flex its manufacturing capabilities during the pandemic, to respond to peak demands. Ajan sees this ability as vital to the post-pandemic environment.
“We have best in class product development. Looking beyond the pandemic, all the learnings and ability to rapidly scale regulatory and manufacturing stand us in good stead for the future. We have built a reliable system of technology transfer and agile manufacturing scale-up to rapidly meet urgent demand.
“We develop novel diagnostics for high unmet medical needs – particularly in near-patient settings. When a patient presents at the ER with respiratory symptoms, you often do not have days to diagnose the cause of their problem. Clinicians and patients need the means to diagnose in a matter of hours. We focus our innovation on faster, better and more efficient diagnostics to drive real-world patient benefits.”
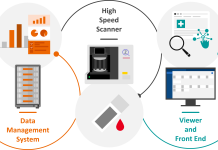
Novacyt’s claim to be industry-leading is backed by the breadth of its portfolio, with more than 300 PCR tests; its ability to demonstrate the highest levels of quality with 95-99% accuracy; and its PROmate® platform, which enables clinicians to give ear-patient PCR diagnostics without the need for expensive laboratory equipment.
The pace of new product development continues – with an eye to the post-COVID world.
“For the moment, our focus is on COVID-19 and coping with the next wave, and we remain fully committed to helping healthcare provides deal with the pandemic,” says Ajan. “But we are also addressing other areas of high unmet need – building on our respiratory expertise – including flu, invasive fungal disease and the transplant sector. If you look at our product development pipeline, we’re planning 26 new tests by the end of 2022, and most will be in the post (non) COVID-19 portfolio.
Future challenges
Ajan says the challenge for the future – for Novacyt and the diagnostics industry in general – is to maintain the high quality of diagnostics that was achieved during the pandemic – putting patients first.
“There’s no argument against earlier clinical diagnosis – either from health economics or from the patient benefit perspective, in achieving better clinical outcomes.
“Yes, for now, the focus is on COVID-19 – and with hindsight, the broader public health community should have recognised that COVID-19 was the most likely source of a global pandemic – but that doesn’t mean we should not focus on other areas of high unmet medical need. COVID-19 has given us the opportunity to maintain the momentum of innovative clinical diagnostics. There has been a revolution in clinical diagnostics. Novacyt may be a relatively small British company, but we’re at the forefront of that revolution.”
Please note: This is a commercial profile