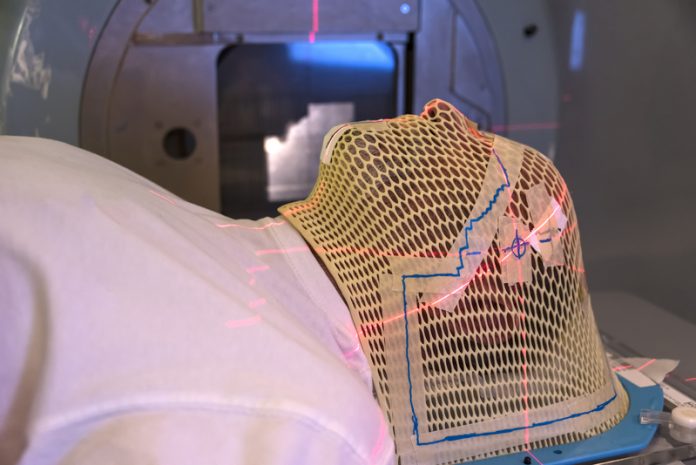
Here, Kristoffer Petersson, MRC Investigator and Group Leader of FLASH Radiation, enlightens us to the benefits of this promising new radiotherapy technique
One in two people in the UK born after 1960 will be diagnosed with some form of cancer during their lifetime. (1) 30-50% of these are expected to receive radiotherapy as part of their treatment. The current level of long-term (longer than 10 years) cancer survivors is 50% but this number is expected to increase if cancer diagnosis and treatment can continue to improve. The success of radiotherapy treatment depends on the ability to deliver a high enough radiation dose to the tumour to eradicate it, while minimising the dose to adjacent normal healthy tissue, to reduce the damage and debilitating side effects caused by the radiation treatment. The quality of life for cancer survivors is as important as the prolongation of life due to treatment. Hence, we need to further improve the level of success of radiotherapy treatment, and the most promising new radiotherapy technique to achieve this, is FLASH radiotherapy.
Recent preclinical studies have shown that FLASH irradiation, which is radiation delivered in a fraction of a second, reduces incidence and severity of radiation side effects compared to conventional irradiation used in clinical practice. However, the treatment effect on tumours is not reduced. (2,3) This is called the “FLASH” effect. The benefit of FLASH radiotherapy has been further shown in veterinarian clinical studies and in the first treatment of a human. (4,5) FLASH radiotherapy uses irradiators with a high radiation output that allows for the treatment to be delivered in fractions of a second, compared to several minutes for conventional treatments.
The short treatment times used in FLASH radiotherapy, often less than 0.1 s, have the added value of minimising treatment delivery uncertainties caused by patient motion during delivery, for example, a reduced risk of missing a lung tumour due to the breathing motion. Carefully implemented, this would allow for smaller volumes of normal tissue being unnecessarily irradiated. Given both the radiobiological advantageous FLASH effect and its potential to “freeze” physiological motion, FLASH radiotherapy has the potential to be an important (r)evolutionary step in cancer treatment. However, we do not yet understand the biology underpinning the FLASH effect. (2,3)
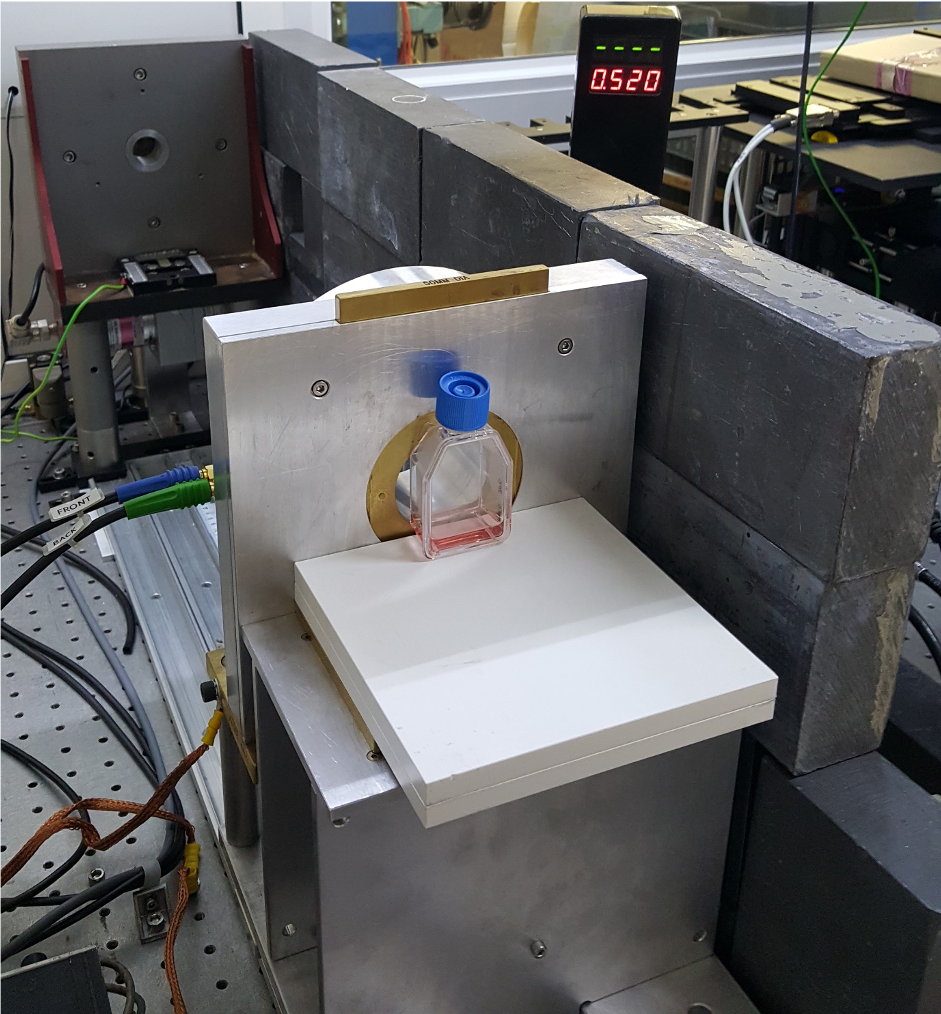
To understand the biology behind FLASH, we work with cell and tissue models (Figure 1). Cancer or normal cells are grown in plastic culture dishes under an environment similar to their physiological conditions. In this way, we can decipher complicate effects using simple experiments. In addition to growing cells into a monolayer, we use cells grown in three-dimensions. Three-dimensional cell cultures, such as cancer spheroids, organoids from normal and cancerous tissues, precision-cut tissue slices, engineered tissues, are also developed to simulate the diversified physiological environments found in humans. For example, though cancer cells are the only cell type used in cancer spheroids, the cells may display different behaviours in different parts of the spheroids due to the oxygen gradient that exists within the 3D structure. To study the interaction of different cell types under FLASH radiation, we use mini-brain and mini-gut derived either from animals or human stem cells. These organoid models, together with precision-cut tissue slices, closely resemble the original tissues but can be grown in a more controllable environment for detailed studies. Recent advances in biomaterial and 3D bio-printing technology allow us to engineer tissues with different structures and environmental cues. These models serve as the platform to bridge the gap between experimental cell culture and the biological environment found in cancer patients.
In addition, we use murine (mouse) models as a research tool to investigate basic mechanisms of cancer development as well as responses of normal tissue and tumour to FLASH radiotherapy. The simplest tumour model we use is the subcutaneous model, in which cell lines initially derived from human tumours (xenograft model) or mouse tumours (syngeneic model) are typically inoculated subcutaneously into the flank of a mouse. Another model that we use is the orthotopic model, which involves the inoculation of tumour cell lines into the corresponding tissue, such as inoculation of brain tumour cells into the mouse brain.
This strategy allows us to assess tumour development in a relevant environment and evaluate treatment effect in a preclinical tumour model that mimics the disease process in humans. With orthotopic models, we can closely monitor and accurately quantify primary tumour growth and metastatic activity. These two models allow us to demonstrate the measurable effects of FLASH radiotherapy on tumour response. Another important aspect of using murine models is to examine the normal tissue toxicity following FLASH irradiation. Radiotherapy can be highly effective, but the dose that can be delivered is limited by the damage it induces in healthy tissue. By evaluating treatment side effects and tumour response in the same model, we are able to measure how the therapeutic index increases for FLASH radiotherapy. This is the most important parameter to increase in cancer therapy.
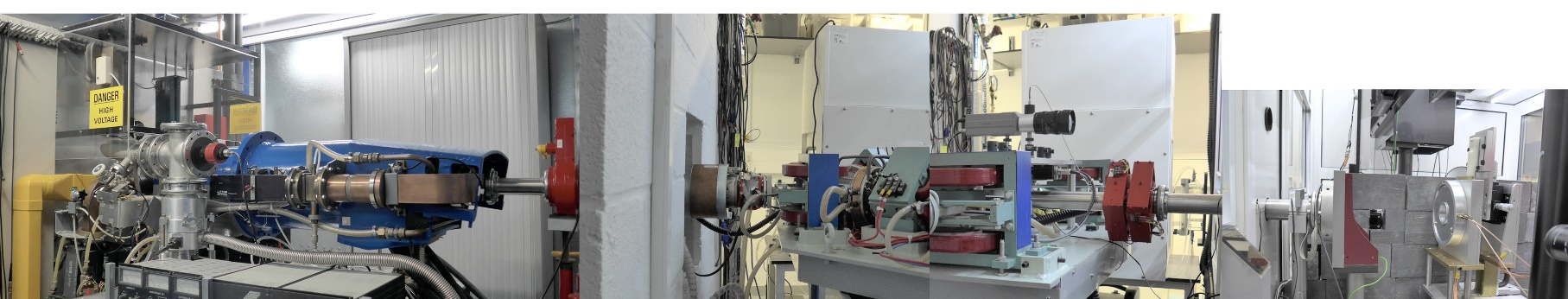
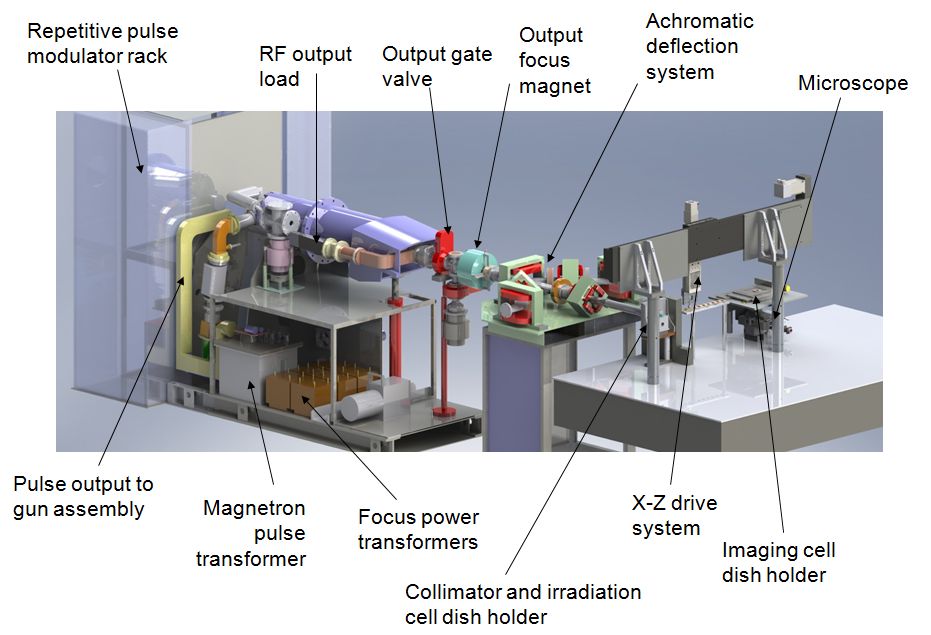
Our research group “Physics and Biology of FLASH Radiation”, at the MRC Oxford Institute for Radiation Oncology, is focused on increasing our understanding of the biological mechanisms responsible for the highly beneficial FLASH effect. By understanding the effect, we will be able to optimise the radiation delivery, to take full advantage of the FLASH effect when introducing the technique in our radiotherapy clinics, potentially benefiting all future patients receiving radiotherapy as part of their cancer treatment. Through funding from sources such as Cancer Research UK’s radiation research network (RadNet) we have state-of-the-art equipment available to us. Specifically, a linear accelerator capable of FLASH radiation dedicated to our preclinical research (Figures 2 and 3) together with a small animal lab and a well-equipped cell and tissue lab.
References
https://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk #heading-Three Cancer Research UK. March 2021
Bourhis, J., et al., Clinical translation of FLASH radiotherapy: Why and how? Radiother Oncol, 2019. 139: p. 11-17.
Wilson, J.D., et al., Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front Oncol, 2019. 9: p. 1563.
Bourhis, J., et al., Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol, 2019. 139: p. 18-22.
Vozenin, M.C., et al., The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin Cancer Res, 2019. 25(1): p. 35-42.
Please note: This is a commercial profile