Jérôme Galon, Director of Research and Head of the Laboratory of Integrative Cancer Immunology at INSERM, explains Immunoscore®, an in vitro diagnostic test that predicts the risk of relapse in colon cancer patients
The past two decades witnessed the placement of the immune system amongst the critical components shaping cancer progression. Alongside the recognition of the key role of specific immune cells, most notably T lymphocytes, came the realisation that monitoring and measuring their levels within tumour lesions could hold prognostic information, which could be leveraged in routine clinical settings.
In 2006, Dr Galon and colleagues demonstrated the importance of the type, density, and location of immune cells within human tumours in predicting colorectal cancer (CRC) patients’ survival and clinical outcome, regardless of tumour progression and invasion1. He defined the so-called “immune contexture”, the most relevant immune-related parameters determining cancer evolution. This previously undescribed strength of the immune reaction changed the understanding of cancer evolution and has important consequences in clinical practice. Indeed, an immune-based theory of cancer evolution was demonstrated2 and the importance of intra-tumour immune T-cells for the patients’ clinical outcome was shown in the primary, as well as metastatic solid tumours3. At the same time, the realisation of the limited accuracy of the current cancer classification, the TNM staging system, which of note doesn’t take into account the immunity of cancer patients, warranted the quest for better prognostic tools.
Surgical removal of the malignancy represents the main or first treatment of colon cancer that has not spread to distant sites (Stage I-III). Chemotherapy is sometimes used after surgery as an adjuvant treatment. Nonetheless, cancer recurrence occurs in a significant percentage of patients and often is found at distant anatomical sites throughout the body (metastases; stage IV). These observations highlight the limitation of the current staging system.
This is the context that led to the development of the Immunoscore®, an in vitro diagnostic test that predicts the risk of relapse in colon cancer patients.
By measuring the density of T lymphocytes populations bearing specific cell surface markers (CD3+ and CD8+) in the centre and at the periphery of the tumour, Immunoscore® represents the first immune-based prognostic tool, which is more powerful than the current TNM classification. The basic concept is simple: a high Immunoscore® reflects high tumour-associated immune cell densities.
Following the first World Immunotherapy Council meeting in 2012, the decision was taken with the Society for Immunotherapy of Cancer (SITC) to initiate an international Immunoscore® consortium featuring 14 centres in 13 countries from North America, Europe and Asia4. The aim of the consortium was to develop, evaluate and validate the consensus Immunoscore® assay in patients with Stage I-III colon cancer. The results of this work were recently published in The Lancet5.
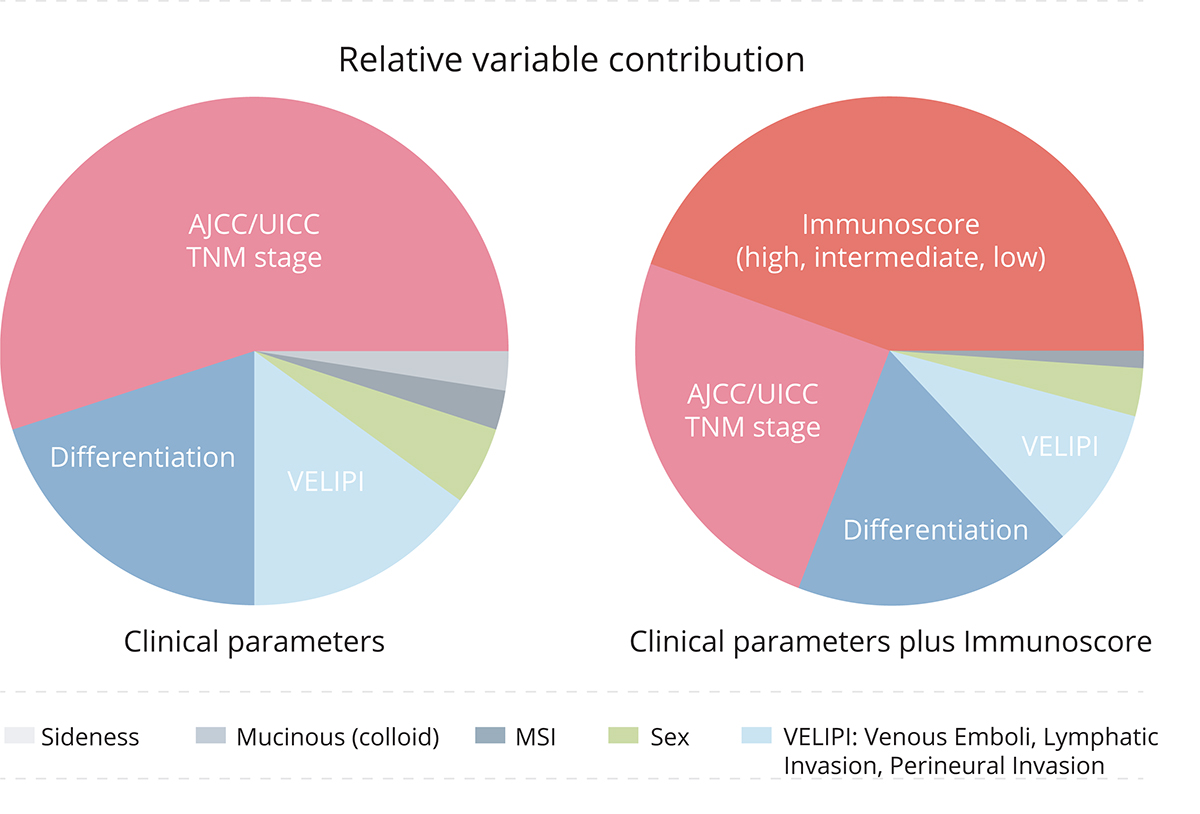
Overall, 2681 colon cancer patients were included in this study. A significant positive correlation was found between the T cell densities in each tumour region and survival, with patients having a high Immunoscore® showing the lowest risk of tumour recurrence. Another scientifically and clinically striking result was that patients with a high Immunoscore® had the longest survival. The analysis of the relative contribution to the risk showed the clear superiority of Immunoscore® in predicting survival compared to all clinical parameters, including TNM and microsatellite instability status (MSI) (Figure 1).
When investigating the strength and dependency of all clinical parameters to predict survival using multivariate analysis (MVA), Immunoscore® and TNM stage were the only significant risk parameters. Thus, the authors supported its implementation and concomitant introduction of an immune component “I” to the TNM (hence becoming TNM-I) cancer classification4.
The consensus Immunoscore® significantly predicted the risk of recurrence and survival in the subgroup of Stage II colon cancer patients. Furthermore, subgroup analysis on high-risk Stage II patients6, showed that nearly 70% of these patients with high-Immunoscore® had a recurrence risk similar to the low-risk patients. This observation reinforces Immunoscore® clinical utility to guide treatment decisions in Stage II patients and to identify patients who could be spared from chemotherapy.
Immunoscore® was further validated in several randomised phase III clinical trials including N0147 study, where it was judged as prognostic in over 1600 stage III colon cancer patients7. The performance of Immunoscore® has today been validated in multiple clinical studies, including more than 5,000 patients, and it’s currently used in routine practice for Stage II and III colon cancer patients. The wide use of Immunoscore® will constitute a step forward to the rightful recognition of the pivotal role played by the immune system in cancer control.
To promote the clinical translation of these findings, Dr Galon co-founded an Immuno-Oncology (IO) diagnostics company, HalioDx, with headquarters in France, and a U.S. subsidiary, HalioDx Inc., for American patients. HalioDx successfully validated the Immunoscore® for in vitro diagnostic use (CE-IVD for European Community countries, and performed in CLIA-certified laboratories). HalioDx was awarded the Seal of Excellence Certificate by the European Commission and with the first-place prize in the Worldwide Innovation Challenge.
Thanks to Immunoscore® and a further set of powerful IO diagnostic tools, HalioDx is now at the forefront of the IO field, thus, leveraging on the recognised role of the immune system in shaping cancer progression. In the future, Immunoscore® should be implemented in clinical decision-making for post-surgical treatment both to refine prognostication and potentially to predict the efficacy of immune checkpoint inhibitors.
1 Galon, J. et al. Science (2006).
2 Angelova M. et al. Cell (2018).
3 Galon J. & Bruni D. Nature Reviews Drug Discovery (2019).
4 Galon, J. et al. J Pathol (2014).
5 Pages, F. et al. Lancet (2018).
6 Galon J. et al. J Clin Oncol (2019).
7 Sinicrope, F. et al. J Clin Oncol (2018).
Please note: This is a commercial profile
Jérôme Galon
Director of Research
Head of the Laboratory of Integrative Cancer Immunology
INSERM
Tel: +33 1 4427 9085