Peter Bretscher, from the University of Saskatchewan looks at whether mouse models of cutaneous leishmaniasis are pertinent for vaccination against and treatment of AIDS, infectious diseases, and cancer
The immune system has two major means of fighting a foreign invader and, on encountering such an invader, employs a decision-making process as to which means to employ. Vaccination as currently practiced increases the tempo and size of the IgG antibody response, and so is effective only against invaders and pathogens susceptible to such antibody.
How general might lessons be of Mouse cutaneous leishmaniasis?
However, clinical observation and experimental studies show that cell mediated immunity is required to contain most cancers, and certain pathogens, such as those causing AIDS, tuberculosis, leprosy, cutaneous and visceral leishmaniasis. Standard vaccination increases the antibody response on infection. This is associated with a downregulation of the cell mediated response. It is for this reason that standard vaccination is ineffective against invaders primarily susceptible to cell-mediated attack.
Mouse cutaneous leishmaniasis is the most intensively researched model of a human disease caused by a pathogen uniquely susceptible to cell-mediated attack. How general might the lessons be from studies in this model?
The two main forms of cell-mediated immunity are cytotoxic T lymphocytes (CTL) and delayed type hypersensitivity (DTH); they are coordinately generated. Their optimal generation can occur in the absence of IgG antibody production. Th1 cells mediate DTH and are protective against Leishmania major, the parasite responsible for cutaneous leishmaniasis.
CTL are protective against most cancers and associated with the generation of Th1 cells. It thus appears plausible that a way of guaranteeing a strong Th1 response against L major is also applicable to guaranteeing a strong CTL response against cancers. Protocols for treating people and animals, who are ill because generating a predominant and ineffective antibody response, instead of a protective cell-mediated response, are also, I will argue, likely to be generally applicable.
The problem for universally efficacious vaccination posed by genetic diversity
Vaccination in use against covid and other pathogens is standard: the same vaccination protocol is given to people of the same age. This might be considered surprising. We and animals are extraordinarily diverse genetically, as I shall indicate, so how come the same amount/form of antigen can protect us all?
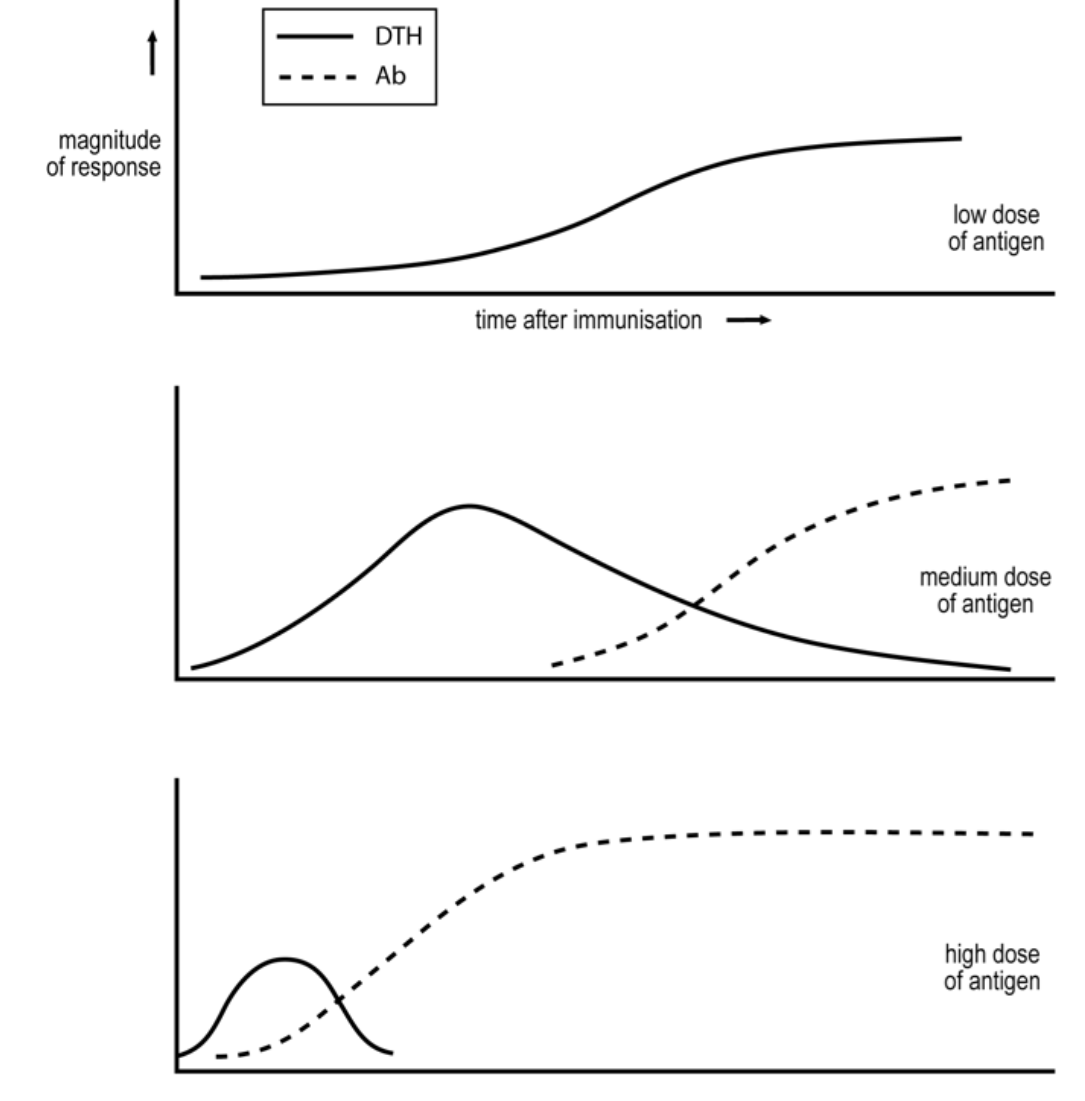
I have discussed elsewhere (eBook 1) how and why different variables of immunization affect the cell-mediated/ antibody nature of the ensuing response. Studies in inbred strains of mice, for example, show the importance of antigen dose. Relatively low doses of antigen are known to generate exclusive, Th1 responses and higher doses transient Th1 responses that decline as antibody is produced and Th2 cells generated. This dose dependence seems to hold for many different types of antigen, and in different animal species, see the figure.
This generality is critical as vaccination, if to be universally effective, must be based upon valid generalisations. A critical problem is that genetics determines what is a low and what is a high dose. A “low dose” in one mouse strain generates an exclusive cell-mediated response but can be a “high dose” in another strain, generating a predominant antibody, Th2 response. Suppose we vaccinate with a very high dose of antigen that induces antibody in all. This will be effective in all against pathogens susceptible to antibody. Such standard vaccination can thus be universally efficacious.
Is universally efficacious vaccination against L major possible?
Infection of mice with a million L major parasites results in “resistant strains” in a stable Th1 response and control of parasitemia; in “susceptible strains”, a predominant Th2 response is rapidly generated and loss of control of parasitemia. We showed that infection of “susceptible mice” with 300 parasites results in a stable Th1 response; infection with a million parasites two months later also resulted in a stable Th1 response and control of parasitemia, i.e. in resistance. This low dose vaccination strategy appears to work in mice against tumors, and in cattle and mice against mycobacteria, responsible for tuberculosis and leprosy, see elsewhere eBook 1.
Can a form of the low dose vaccination strategy be developed that is universally efficacious despite genetic diversity of the vaccinated? We thought this could be a major problem and sought a way to transcend it.
Valid generalisations must underlie any universally efficacious vaccination strategy. We sought to examine in diverse mouse strains whether the dependence of the Th1/Th2 phenotype of the response always depends upon the parasite number employed for infection. We found that infection with relatively low and high numbers of parasites resulted respectively in stable Th1 responses and in responses with a significant Th2 component. We could thus define for a mouse strain a transition number, Nt: infection with a number below Nt resulted in a stable Th1 response; infection with a number above Nt resulted in time in a response with a substantial Th2 component. The higher the number was above Nt the faster was the generation and the size of the Th2 component. Remarkably, the value of Nt varied in our study over a 10,000 fold range. Nt was 500 parasites in the most susceptible strain and 5,000,000 in the most resistant strain. This study led us to two conclusions. Genes dramatically affect the Th1/Th2 phenotype of the ensuing response. Most importantly, this phenotype generally does depend on the parasite number employed in the infection. Suppose we infected diverse mice with 250 parasites, below the transition number for any individual. As these parasites are alive and replicate, they would be expected to generate Th1 responses and Th1 imprints in all mice, thus constituting universally efficacious vaccination. We refer to this as the very low vaccination strategy to achieve universally effective protection.
It appears this strategy may be the basis of leishmanization as carried out in the Middle East. Parents expose the bottoms of their babies to infected sandflies, the insect vector for the parasite, and thus avoid the formation of pockmarks on the face later in life, that are socially disadvantageous. Vaccination with live parasites will be unacceptable in the west. I suggest vaccination with very low doses of slowly replicating vectors, encoding immunodominant parasite antigens, could be employed.
Treating visceral leishmaniasis
It is standard practice in tropical, endemic areas to treat otherwise lethal visceral leishmaniasis with a short course of drugs that kills the parasite. We and others showed such treatment modulated the immune response from a mixed Th1/Th2 to a Th1 phenotype. Treated individuals are resistant to becoming ill on reinfection. We argue that this modulation is because the drug, in killing the parasite, lowers the antigen load. I suggest treatment of HIV infections and of cancer can similarly be treated by harnessing protective, Th1 immunity, by reducing the antigen load by one means or another.
Please note: This is a commercial profile
© 2019. This work is licensed under CC-BY-NC-ND.