Sean E. Lawler PhD from Brown University Cancer Center, walks us through our friends oncolytic, or in other words, using viruses to cure cancer
Imagine a drug that could selectively destroy cancer cells with absolute precision, leaving normal, healthy cells untouched, resulting in the safe and total elimination of the cancer, and complete cure. Scientists are trying to make that dream come true by using designer viruses to specifically target and destroy tumour cells. This approach is based on the idea that genetic engineering of viruses (usually thought of as dangerous pathogens) could be used to make them into cancer killers by infecting and replicating only in tumour cells and lysing them in the process, leading to cancer elimination, hence the name oncolytic virus (OV). This has now become a major field in cancer therapy, and a range of different viruses are now being tested against many cancer types, in the lab and in the clinic.
Oncolytic viruses (OV)
It has long been known that injecting pathogens into tumours can lead to cures dating back to the 19th century. However, it was not until the late 1980s that the possibility of engineering viruses to render them tumour selective became technically possible. This has led to the development of approaches based around many different virus genetic backbones. In 2015, the OV field got a great boost following approval by the U.S. FDA of an engineered human herpesvirus (T-VEC, Imlygic) for the treatment of advanced melanoma. T-VEC is injected at high numbers into the tumour and has been engineered to remove some of the genes that cause virulence in normal cells, and also to express immunostimulatory genes to recruit immune cells. The main question in the field now is whether such an approach can be used more broadly in other cancer types, including devastating cancers like brain tumours.
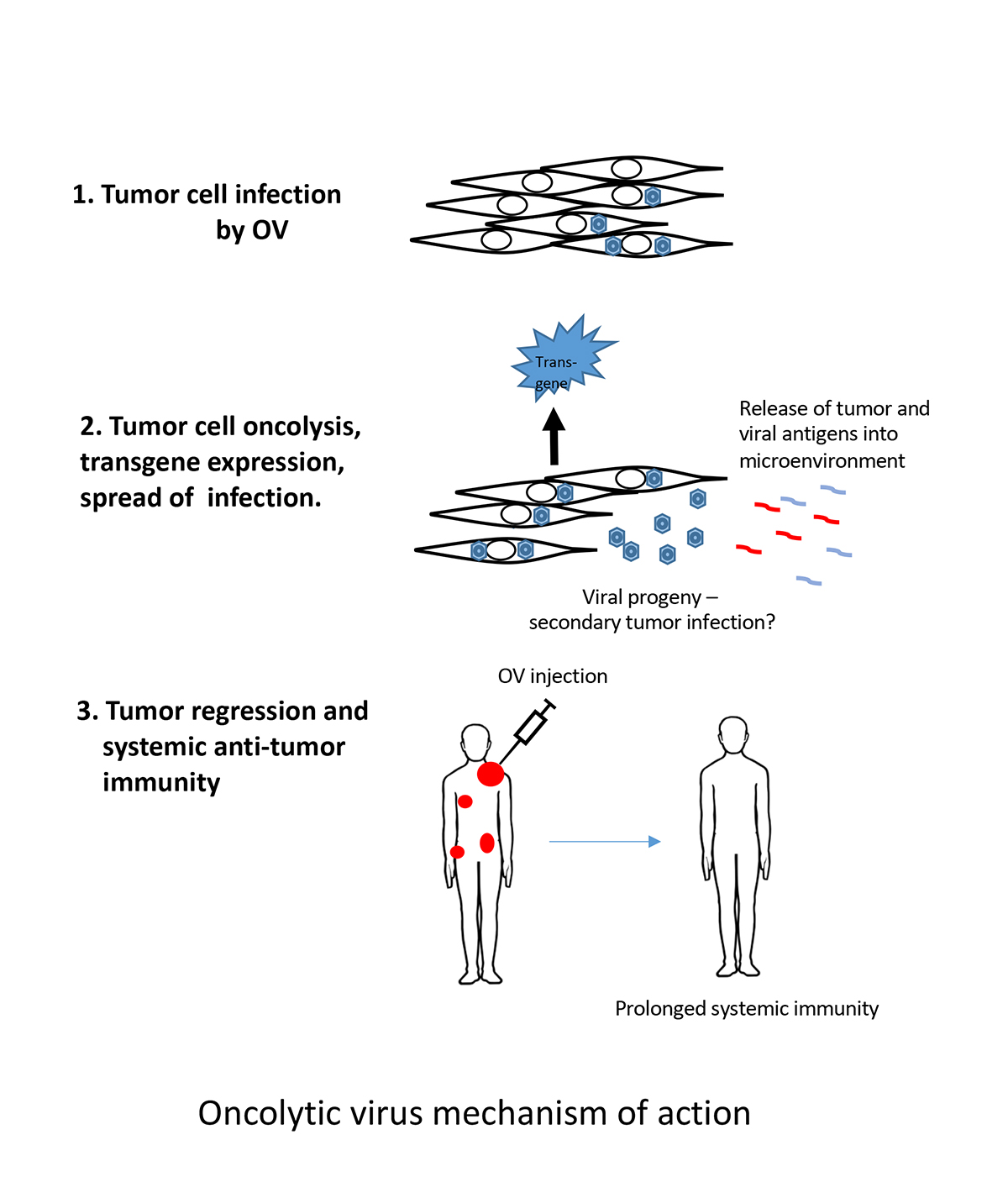
In theory, an OV-infected tumour would eventually burst, releasing thousands of viruses which would then go on to infect more tumour cells, until the tumour is gone. In reality, the spread of OVs is rapidly blocked because cells can detect viruses and prevent them from spreading.
These sensing mechanisms lead to the recruitment of virus hunting immune cells to the tumour, resulting in a breakdown of the immune-suppressive mechanisms that tumours typically use to hide themselves from the immune system. This may essentially “uncloak” the tumour allowing an anti-tumour immune response. Thus, our current view of OVs is that they act in a dual fashion – an initial phase of selective tumour cell killing, which is followed by the more important uncloaking step allowing anti-tumour immunity to develop (see figure).
This type of approach would be very useful in brain cancers like glioblastoma because tumour cells invade normal brain tissue well beyond the main tumour mass and, therefore, it is impossible to surgically remove all of the tumour cells, which are entwined within the structures of the normal brain. These tumours are also notoriously immunosuppressive – but when a living virus is detected, even within the tumour mass, the body is still capable of recognising and eliminating the pathogen, providing a window of opportunity for the immune system to see the cancer itself. Thus, OVs work together with our own bodies to unleash the cure within – our own immune systems, which can, in some cases lead to the complete cure we seek. Trials to date have a great safety record, and some remarkable responses have been reported. However, it seems that generally, OV replication is limited and that effective treatment requires an immune response against the virus, and then to the tumour.
To create an OV, human viruses like herpes, adenovirus, poliovirus and measles are commonly used – and these may be engineered to reduce virulence towards normal cells, and sometimes have modified coat proteins that allow tumour-selective cell entry. Other viruses widely used may be from other non-human species, such as vesicular stomatitis virus and Newcastle disease virus. The newer generations of OVs are generally more potent or may be “armed” to carry additional transgenes to stimulate an immune response.
Other approaches include combination therapies where an OV is administered alongside another drug. For example, after promising preclinical studies in mouse cancer models, combinations with immune checkpoint blockade are now being tested clinically in the hope that a two-pronged approach of local immune activation and systemic immunotherapy may improve outcomes in human patients. Typically, the virus is injected directly into the tumour – in brain cancer, this is done at the end of surgery, by injecting the OV at multiple sites around the injection cavity, although OVs that can be injected systemically are also in development.
The future of research in the OV field
At this moment in time, a wide array of viruses, combinations and administration routes are being investigated. Although the field is awaiting the next major breakthrough, there is a sense that learning which patients respond, and which viruses to use, and how to use them may provide better outcomes. For example, a herpes-based OV was recently approved for use in recurrent glioblastoma in Japan – the key in this approach seems to have been to perform repeat injections of the OV over a period of months.
Improved viruses, in terms of tumour selectivity and potency, and optimised combinations with other immune therapies may lead to further improvements in patient outcomes. Given the increasing array of OVs in development, as well as other immune-stimulatory agents, the challenge in the field going forward will be to successfully identify the OVs and combinations that will be the most effective for patients, particularly those with tumours that are resistant to other therapies. Although OVs have not fulfilled the dream of tumour selective destruction, they open the door to the most precise tool of all – our own immune system. Learning how to perform this trick broadly across patients would bring OVs to the forefront of cancer medicine.
Please note: This is a commercial profile