In this report, Professor Peter H. Santschi, Texas A&M University at Galveston, highlights the challenges when containing iodine in a solid phase for radioactive waste disposal
Silver-impregnated zeolite (AgIZ) has been used for removing radioiodine from contaminated groundwater and nuclear waste streams and the worldwide inventory of such secondary waste is rapidly increasing. To contain long-lived radioactive iodine (16 Mio year half-life of 129I) from nuclear waste, various methods have been employed, with various success.
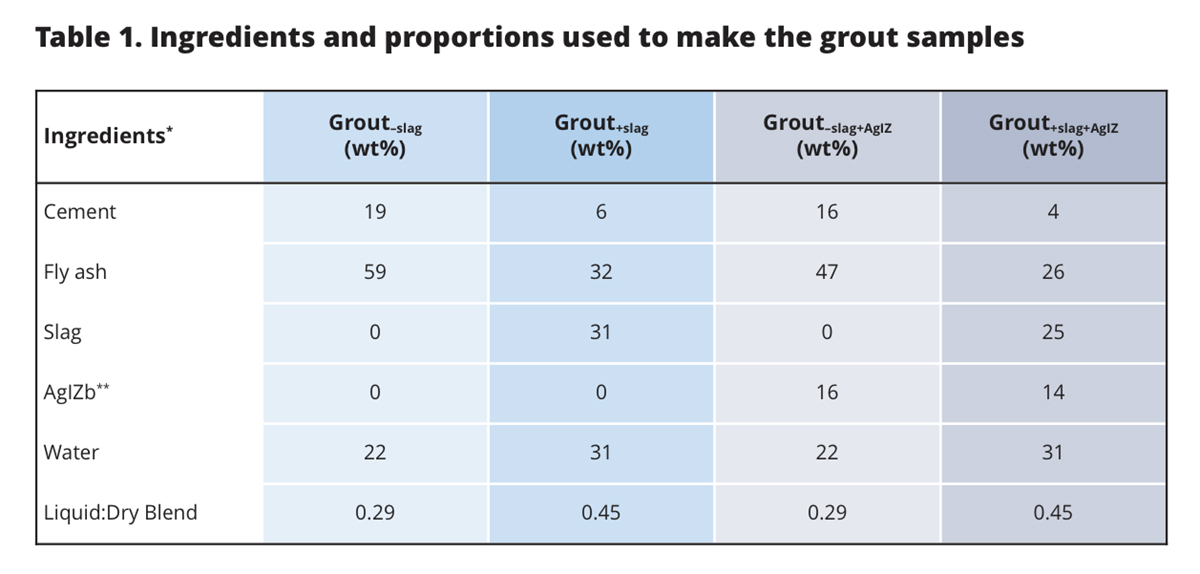
One of the ways 129I can be immobilized is through the formation of insoluble silver iodide solid, AgI, which is only possible in the presence of oxygen (Fig. 1). The silver technology is commonly deployed by coating silver on zeolites, AgZ, and then putting it contact with the aqueous or gaseous 129I waste stream. The objective of a recent study [Kaplan et al., 2019] was to quantify the effectiveness of two grout waste formulations for disposing of the used AgIZ (Table 1). The grout formulations were with and without slag, an ingredient used to reduce porosity and to create a strong chemically reducing environment to immobilize redox reactive contaminants).
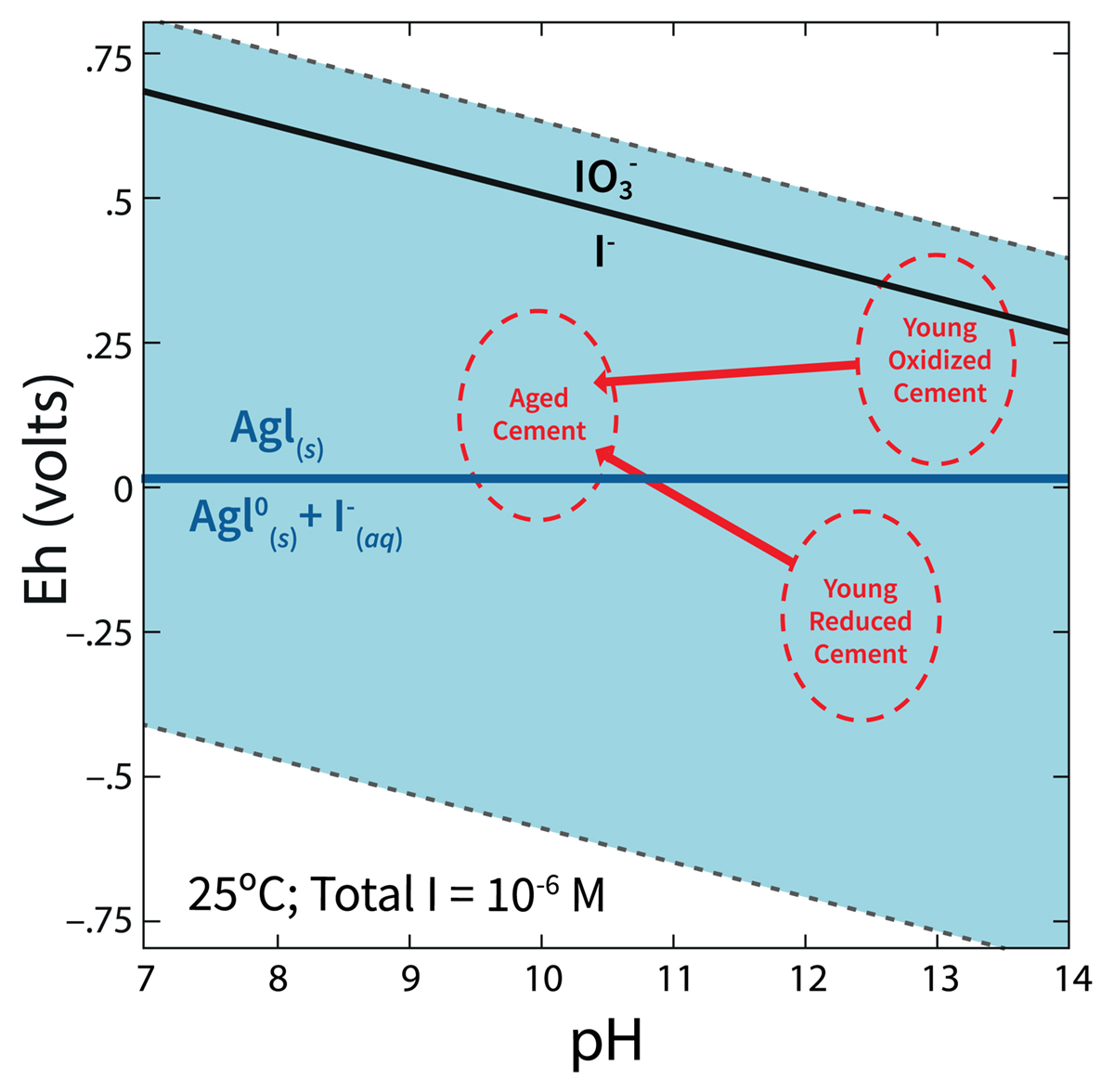
A second objective was to determine the iodine speciation leached from AgIZ encapsulated in grout. Iodine commonly exists concurrently as three species in the same environment: iodide (I-), iodate (IO3-), and organo-iodine (org-I). To predict the toxicity and the rate at which 129I travels, it is important to know the iodine speciation in the system. A 60-day kinetic batch experiment demonstrated that AgIZ encapsulated in slag-free grout was extremely effective at immobilizing iodine and silver, a potential non-radioactive carcinogen.
However, AgIZ encapsulated in slag-containing grout, the most common type of grout used for low-level radioactive waste disposal, was entirely ineffective at immobilizing iodine. While the slag-free grout with AgIZ released only 3.3 µg/L total iodine into the contact solution, the slag-containing grout released 19,269 µg/L total iodine. Based on thermodynamic calculations, the strongly reducing conditions of the slag-containing system (Eh was -392 mV) promoted the reductive dissolution of the AgI solid, forming a dissolved form of silver, Ag0(aq), and releasing iodide into the aqueous phase. The slag-free grout system was maintained under more oxidizing conditions (Eh was 439 mV) and a minimal amount of iodine was released from the grout.
** AgIZ is the Ag-zeolite (AgZ) amended with I-.
In both grout systems, the aqueous iodine, originally added to the AgZ as iodide, was composed primarily of iodide and org-I, and essentially no iodate was detected. More organo-I was detected in the slag-free than the slag-containing grout system, because the high redox potential of the former system was more conducive to the formation of oxidized I species, such as I2 and HIO, which may be intermediates in the covalent bonding of iodine with organic carbon in grout. Spectroscopic analysis (X-ray Absorption Near Edge Structure analysis) of the two AgIZ-grout samples indicated that the iodine existed exclusively as silver iodide.
Together, these results show that subsurface grout disposal of AgIZ waste should be done under oxidizing conditions and that radioiodide released from AgIZ can undergo speciation transformations (leading to organo-formation) that have important implications on subsequent mobility and estimated risk. [Kaplan, D.I., Price, K., Xu, C., Li, D., Lin, P., Xing, W., Nichols, R., Schwehr, K.A., Seaman, J.C., Ohnuki, T., Chen, N., Santschi, P.H. 2019. Iodine Speciation in a Silver-Amended Cementitious System. Environment International, in press]
Please note: This is a commercial profile
Peter H. Santschi
Professor
Texas A&M University at Galveston, TX
Tel: +1 409 539 1007