Tara M. DeSilva from the Department of Neurosciences, Cleveland Clinic, walks us through regenerative therapies for multiple sclerosis, including comments on clues from studying normal brain development
Approximately 3 million people worldwide are living with multiple sclerosis (MS), a leading cause of neurological disability in young adults. [1] MS is a neuroinflammatory demyelinating disease characterised by lesions in the brain, spinal cord and optic nerve (i.e., central nervous system, CNS) that are detected using magnetic resonance imaging. Lesions in MS are multifarious, giving rise to heterogeneous symptoms depending on their location.
What is multiple sclerosis?
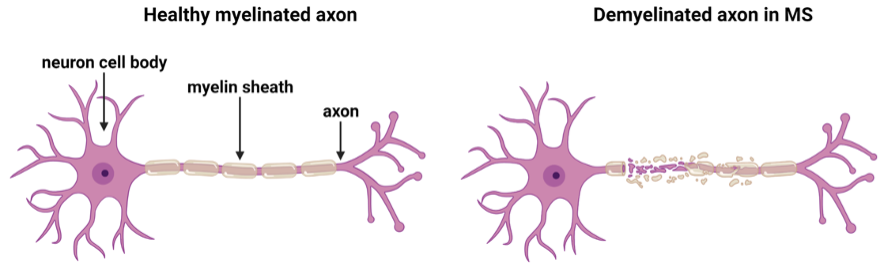
In MS, the immune system becomes abnormally activated, entering the CNS, and facilitating damage to myelin, the insulating sheath around nerve fibres (axons) causing demyelination (Figure 1). Axons are responsible for conducting action potentials essential for transmitting information to other neurons throughout the CNS. The myelin sheath regulates the speed of nerve conduction and maintains the health of the axon. Thus, damaged myelin, not only slows the propagation of nerve impulses giving rise to disability in MS, but chronic demyelination perpetuates axonal loss, which is a key component of disease progression and permanent neurological disability.
Why are regenerative therapies important for multiple sclerosis?
Current therapies for MS target immune cells by limiting their numbers or inhibiting their entry into the CNS. Although these immunomodulatory strategies decrease relapses and improve quality of life early in the disease course, ultimately disease progression ensues. Regenerative strategies are essential to overcome this barrier, with early intervention being critical to rescue axonal integrity and overall neuronal function. One such strategy involves remyelination, or the formation of new myelin sheaths from cells that produce it, called oligodendrocytes. In demyelinating lesions in MS, early-stage myelin-producing cells called oligodendrocyte progenitor cells (OPCs) proliferate as a prerequisite for remyelination but fail to rebuild the insulation around axons. This is in contrast to normal development when OPCs become mature oligodendrocytes and successfully form myelin sheaths around axons. Mechanisms that drive OPC maturation to form myelin are, therefore, a major focus for remyelinating therapies in MS.
How can a developmental process advance therapeutic strategies for multiple sclerosis?
The DeSilva laboratory’s recently published study [2] uncovered an important mechanism of developmental myelination: regulation of OPC number. Cutting-edge neuroimaging and genetic labelling techniques were utilised to track and visualise cellular interactions using in vivo models of development. Microglia (Figure 2, green) were found engulfing OPCs (Figure 2, red) through a phagocytic process during a restricted developmental window before the onset of myelination. Microglia are a specialised population of cells in the CNS that respond to inflammatory processes in the brain. They have mainly been studied in the context of contributing to inflammation and cellular damage as well as clearing cellular debris in disease states.
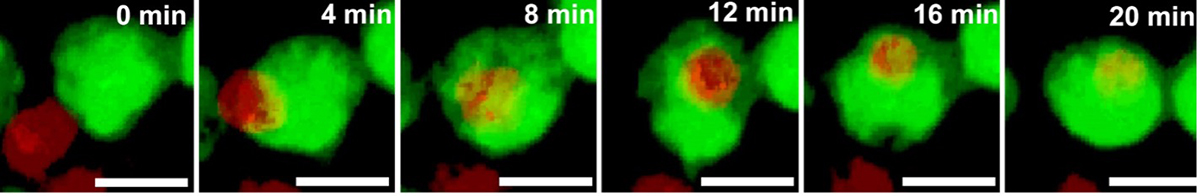
More recent studies have demonstrated their role in engulfing neurons during development as well as structures that form connections between neurons, called synapses. This is the first study, however, documenting their role in phagocytosing OPCs to regulate myelination. More than 30% of OPCs were engulfed by microglia, indicating the substantial nature of this developmental process. It was also found that the population of microglia engulfing OPCs was amoeboid in shape (activated state), but as OPCs matured to form myelin and ensheath axons later in development, the microglia transitioned to a ramified or resting state.
To further explore the mechanisms regulating the cue for engulfment, time-lapse imaging was performed to document live cellular interactions between microglia and OPCs.
Engulfment for the purpose of cell degradation is usually triggered by the expression of “eat me” signals in cells that are stressed or dying. Interestingly, the majority of OPCs being engulfed by microglia did not express markers of cellular stress. Thus, microglia engulf OPCs during their pre-myelinating state when they are viable and healthy, suggesting that this process is necessary to reduce OPC number and ensure proper myelination. To understand the impact of OPC removal by microglia on myelination, the ability of microglia to engulf OPCs was blocked by deleting a key signalling factor from microglia called the fractalkine receptor. This did not change the morphology or number of microglia but did reduce their ability to phagocytose OPCs resulting in an increased number of oligodendrocytes. Deleting the fractalkine receptor also resulted in the formation of thinner myelin sheaths, suggesting that overcrowding of oligodendrocytes impaired the efficiency of the myelination process. These results indicate that microglia perform a critical role in regulating the number of OPCs to facilitate proper myelination with potential ramifications for remyelinating therapies in MS. Studies in human post-mortem brains from individuals with MS show that OPCs are present in chronic lesions, but do not properly reform myelin. [3] The presence of newly generated OPCs in the adult MS brain represents a developmental process that requires refinement by microglia to initiate proper myelin formation.
These data suggest that reprogramming of microglia to a developmental state to regulate OPC number may provide a therapeutic strategy for remyelinating therapies.
References
- Walton, C., et al., Rising prevalence of multiple sclerosis world-wide: Insights from the Atlas of MS, third edition. Mult Scler,
2020. 26(14): p. 1816-1821.
- Nemes-Baran, A.D., D.R. White, and T.M. DeSilva, Fractalkine-Dependent Microglial Pruning of Viable Oligodendrocyte Pro- genitor Cells Regulates Myelination. Cell Rep, 2020. 32(7): p. 108047.
- Chang, A., et al., Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. New England Journal of Medicine, 2002. 346(3): p. 165-173.
Please note: This is a commercial profile
© 2019. This work is licensed under CC-BY-NC-ND.