Efficient and decentralised production of sustainable fuels for today’s transport infrastructure is discussed here by the Technical University of Denmark, who call for the development of highly active catalysts
Society needs alternative energy production systems. Sustainable synthesis of fuels in decentralised units constitutes a cheap, effective and stable solution, meeting needs from industry and users. Methanol (CH3OH) is a highly used chemical with a large variety of applications. [1] Furthermore, it has been suggested to use methanol as transportation fuel [2] as an alternative to conventional petroleum-based fuels. Industrial scale synthesis of methanol from syngas is typically carried out at elevated temperatures and pressures over a Cu/ZnO/Al2O3 catalyst between 200-300°C and 50-100 bar. [3] This pressure level is relevant for industrial applications, but such processes involve large scale production units, high operational and investment costs. Decentralised units would constitute cheap and mobile facilities that produce liquid fuel (methanol) from solar- or wind-generated hydrogen. A catalyst suitable for such a decentralised application requires different conditions compared to known catalysis of methanol synthesis. If methanol has to be produced in decentralised units, a low temperature and pressure process needs to be developed. A schematic presentation of this dream scenario is illustrated in Figure 1. [4]
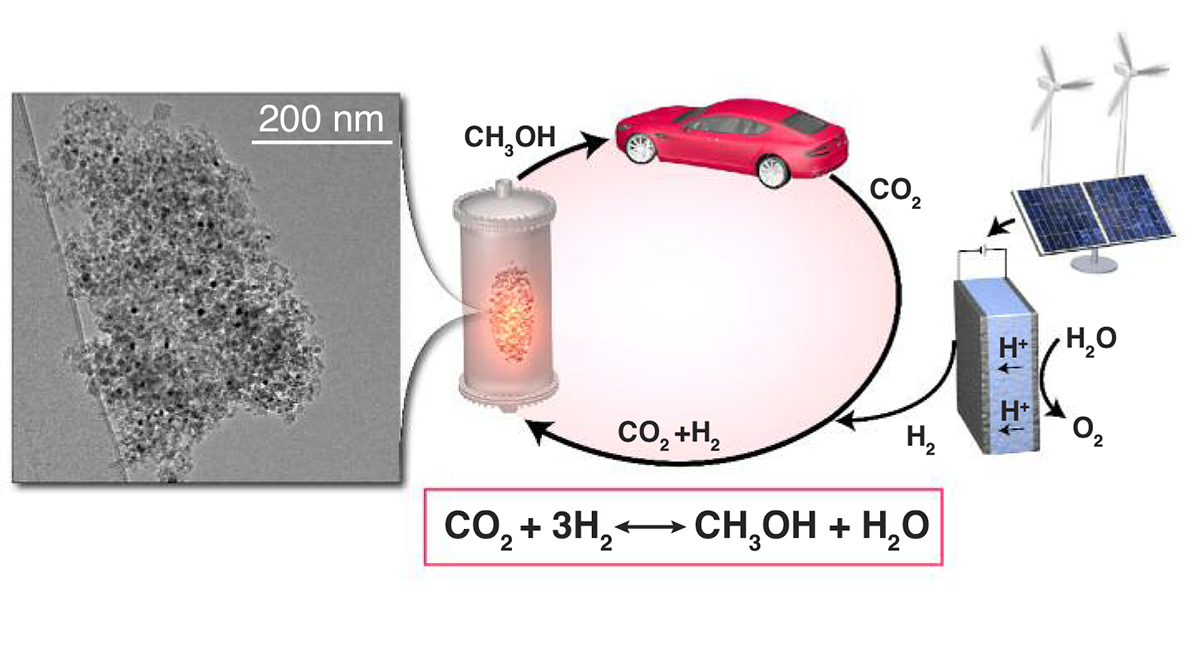
In a collaboration between the Villum Center for the Science of Sustainable Fuels and Chemicals at DTU and the electron microscopy facility at Nanolab DTU, several bi-metallic intermetallic alloy catalysts have been studied and found highly active for ambient pressure methanol production directly from CO2 and H2. By employing in situ high-resolution electron microscopy (HRTEM) and x-ray based methods, such as absorption spectroscopy (XAS) and diffraction (XRD), an important understanding regarding the correlation between the catalytic activity and the catalyst elemental composition and structure has been obtained. This knowledge is essential to further optimise and develop novel catalysts for sustainable methanol production.
During the synthesis of methanol from CO2 and H2, several competing reactions can occur. The most devastating is the reverse water gas shift (rWGS) reaction, where CO and H2O are formed and methanation, where CH4 and H2O are formed. In both cases, the formed H2O pushes the methanol equilibrium towards the reactants and, thus, lower the overall methanol yield. Furthermore, CH4 and CO can deactivate the catalyst through restructuring or carbon poisoning through dissociation. Thus, the overarching goal has been to find highly selective catalysts i.e. a catalyst that works efficiently for the methanol reaction and prohibits competing reactions.
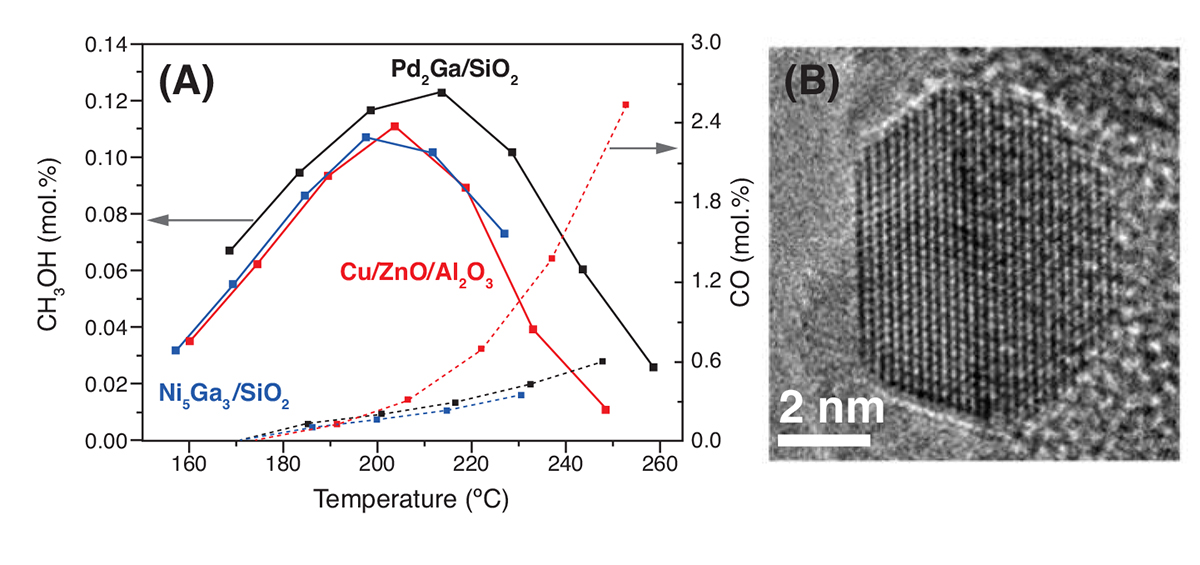
This is the case for Pd2Ga [5,6] and Ni5Ga3 [7,8] catalysts, which has been found by Fiordaliso et al. and Studt et al. and Sharafutdinov et al., respectively, to be as active or more active than Cu/ZnO/Al2O3 catalyst. Figure 2 (A) illustrates the methanol and CO yield vs. temperature for all three catalytic systems. Both Pd2Ga and Ni5Ga3 produces less CO than the conventional Cu/ZnO/Al2O3 at temperatures above 200°C.
In order to, fully understand the structure-activity relationship, complementary characterisation methods were employed during both catalyst formation and catalytic tests. In situ XRD and XAS were used to follow the catalyst’s average structural and electronic changes during synthesis and reaction conditions. [9] Nanoscale imaging and spectroscopy of catalysts in a gaseous environment were performed in an environmental transmission electron microscope (ETEM). As an example Figure 2 (B) shows a high-resolution electron microscopy (HRTEM) micrograph of a single nanoparticle acquired during alloy formation at 700°C in 1 mbar H2. From HRTEM nanoparticle shape and size as well as crystallinity and exposed facets can be related to the catalytic activity, as well as ensemble averaging characterisation methods such as XRD and XAS.
The approach of combining complementary characterisation tools with catalytic test has proved to be a comprehensive and powerful method for studying intermetallic catalysts for CO2 hydrogenation to methanol. Furthermore, the developed methodology would be applicable to a wide range of catalytic systems.
References
- Hansen, J.B., Nielsen, P.E.H.: Methanol synthesis. Handb. Heterog. Catal. 2920 (2008).
- Olah, G.A.: Towards oil independence through renewable methanol chemistry, (2013).
- Behrens, M., Studt, F., Kasatkin, I., Kühl, S., Hävecker, M., Abild-Pedersen, F., Zander, S., Girgsdies, F., Kurr, P., Kniep, B.-L., Tovar, M., Fischer, R.W., Nørskov, J.K., Schlögl, R.: The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science. 336, 893-7 (2012). doi:10.1126/science.1219831.
- Dahl, S., Chorkendorff, I.: Solar-fuel generation: Towards practical implementation. Nat. Mater. 11, 100-101 (2012). doi:10.1038/nmat3233.
- Fiordaliso, E.M., Sharafutdinov, I., Carvalho, H.W.P., Grunwaldt, J.-D., Hansen, T.W., Chorkendorff, I., Wagner, J.B., Damsgaard, C.: Intermetallic GaPd 2 nanoparticles on SiO 2 for low pressure CO 2 hydrogenation to methanol: catalytic performance and in situ characterization. ACS Catal. 5, 5827-5836 (2015). doi:10.1021/acscatal.5b01271.
- Fiordaliso, E.M., Sharafutdinov, I., de Carvalho, H.W.P., Kehres, J., Grunwaldt, J.-D., Chorkendorff, I., Damsgaard, C.D.: Evolution of intermetallic GaPd2/SiO2 catalyst and optimization for methanol synthesis at ambient pressure. Accept. Publ. Sci. Technol. Adv. Mater. (2019).
- Studt, F., Sharafutdinov, I., Abild-Pedersen, F., Elkjær, C.F., Hummelshøj, J.S., Dahl, S., Chorkendorff, I., Nørskov, J.K.: Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 6, 320-324 (2014). doi:10.1038/nchem.1873.
- Sharafutdinov, I., Elkjær, C.F., Pereira de Carvalho, H.W., Gardini, D., Chiarello, G.L., Damsgaard, C.D., Wagner, J.B., Grunwaldt, J.-D., Dahl, S., Chorkendorff, I.: Intermetallic compounds of Ni and Ga as catalysts for the synthesis of methanol. J. Catal. 320, 77-88 (2014). doi:10.1016/j.jcat.2014.09.025
- Clausen, B.S.: Combined (Q)EXAFS/XRD: Technique and Applications. Catal. Today. 39, 293-300 (1998). doi:10.1016/S0920-5861(97)00115-6.
Christian Danvad Damsgaard
Associate Professor
DTU Nanolab/DTU Physics,
Technical University of Denmark
Tel: 45 4525 6487
Professor Jakob Birkedal Wagner
Head of Nanocharacterization Section
DTU Nanolab,
Technical University of Denmark
Tel: +45 4525 6471
Please note: This is a commercial profile











