Tobias Deuse, Cardiac Surgeon and Scientist at University of California San Francisco, reveals to us the future of regenerative cell therapeutics
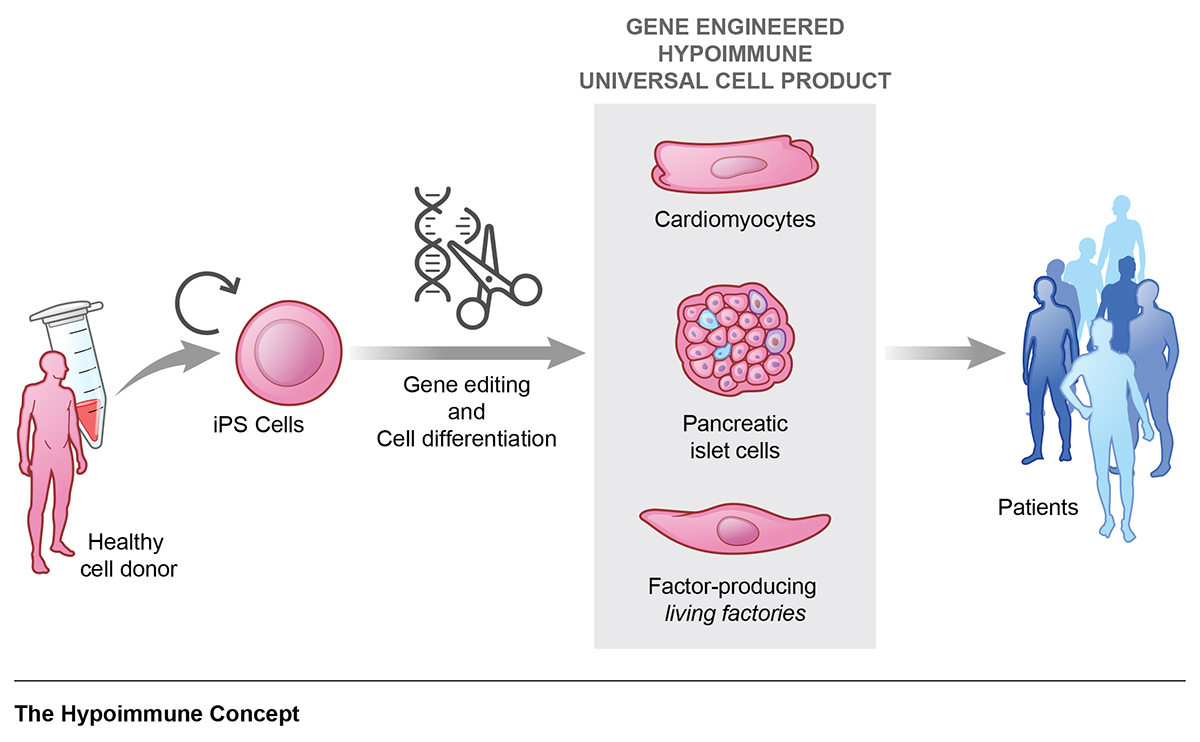
The concept of using cells as medicines has opened many opportunities to treat diseases for which there are no good therapeutic options available. The basic idea is to replace cells in the body that are diseased or have been destroyed in a disease process. We are currently in the very early stages of this new era and many different applications are being explored. The process of making enough specialised cells to restore the function of an organ is quite lengthy and complex. Induced pluripotent stem cells (iPSC) are reprogramed cells to a developmentally early pluripotent stage that can serve as a virtually unlimited source for cells that can be differentiated into a specific cell type. Protocols for the differentiation of neural progenitors, retinal pigment epithelial cells, pancreatic islet cells, and cardiomyocytes have been established, among others. Cells have to be purified and meet certain quality criteria before they can be released for clinical use. All this process has to be done under good manufacturing practice (GMP) conditions.
The immune system as a barrier to cell transplantation
Transplanted cells, just like transplanted tissues or organs, are subject to rejection if they are generated from a different individual. The immune system recognises them as “foreign” and work from our lab has shown how thorough the immune system is in this respect1,2. To avoid rejection, current approaches for clinical trials focus on making patient-specific cell therapeutics. However, this is a labour-intensive and costly strategy and the quality of cells generated can vary significantly. The value of these studies for proof-of-concept is indisputable, but for the reasons mentioned, patient-specific, so-called autologous concepts will most likely not become widely available options.
Immune-evasive cells can be the solution
The idea of doing this whole process only once and getting an off-the-shelf cell product that can be universally used for all patients is intriguing. It would allow streamlined manufacturing of large amounts of cells with defined quality measures at lower costs. The immune barrier, however, prevents this allogeneic approach since the cells would have been derived from a different donor and would consistently be rejected in all patients. Therefore, much interest has been captured by new gene-editing strategies, which can markedly reduce the immunogenicity of cells and help them escape immune recognition. After trialling many different combinations in our lab, we came up with a 3-edit concept that reliably establishes immune evasiveness from all kinds of adaptive and innate recipient immune cells3. Such “hypoimmune” cells showed unrestricted long-term survival in allogeneic mouse models and were protected from immune killing in human in vitro settings4. A translation of allogeneic hypoimmune cells to clinical grade could potentially open the door to large-scale manufacturing of universal cell therapeutics.
Preclinical studies establish proof-of-concept
After we demonstrated the efficacy and reliability of the immune evasion concept in different types of cells including endothelial cells, smooth muscle cells and cardiomyocytes, we wanted to explore whether we can treat diseases in preclinical settings. We picked three major diseases involving different organ systems in mice with established models and transplanted allogeneic hypoimmune cells5.
Hypoimmune endothelial cells facilitated limb preservation in animals with critical limb ischemia and hypoimmune cardiomyocytes reduced heart failure after myocardial infarction. In addition, we could show that hypoimmune endothelial cells can be turned into living factories to produce alpha-1-antitrypsin in mice with genetic deficiency and prevent emphysematous lung disease. This study demonstrated the versatility of the hypoimmune concept for a variety of different diseases and encourages the development of clinical-grade products for human trials.
Roadmap of diseases
Restoration of cell or organ function faces different levels of complexity for different diseases. Remuscularization of the heart with injected cardiomyocytes requires orthotopic engraftment within the heart muscle with electrical and mechanical coupling to the host tissue. Cell transplantation for endocrine diseases or genetic single factor deficiencies, on the other hand, can be done heterotopically with cells being injected into any well-vascularized tissue and cells don’t have to directly interact with surrounding tissue other than sensing and producing its factor. Diabetes mellitus, factor IX-deficiency, and alpha-1-antitrypsin deficiency are examples for diseases amenable for more straightforward implementation of hypoimmune-based regenerative cell therapy. For autoimmune diseases like Hashimoto’s immune thyroiditis that are primarily driven by antibody-mediated cell destruction, we have developed additional immune escape mechanisms to protect cells from antibodies.
Impact on health equity
To facilitate the broader adoption of cell therapeutics, affordability is critical. Access of underserved communities to healthcare is often restricted because of higher proportions of patients having no or limited insurance. Especially when it comes to costly or lengthy treatments, low-income individuals are less likely to be treated. This health disparity becomes even more pronounced as the prevalence of risk factors for chronic diseases is higher and early detection of diseases is lower among low-income and minority groups. Much hope is, therefore, put on the successful development of affordable, universal, off-the-shelf hypoimmune cell therapeutics for all patients in need.
References
- Deuse, T., et al. De novo mutations in mitochondrial DNA of iPSCs produce immunogenic neoepitopes in mice and humans. Nat Biotechnol 37, 1137-1144 (2019).
- Deuse, T., et al. SCNT-derived ESCs with mismatched mitochondria trigger an immune response in allogeneic hosts. Cell Stem Cell 16, 33-38 (2015).
- Deuse, T., et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol 37, 252-258 (2019).
- Deuse, T., et al. The SIRPalpha-CD47 immune checkpoint in NK cells. J Exp Med 218(2021).
- Deuse, T., et al. Hypoimmune induced pluripotent stem cell-derived cell therapeutics treat cardiovascular and pulmonary diseases in immunocompetent allogeneic mice. Proc Natl Acad Sci U S A 118(2021).
Please note: This is a commercial profile