Prof Dr Susanne M Bailer, Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB, explains the possibilities of an oncolytic virus platform technology
Cancer is a disease that affects most of us, directly or indirectly. In 2018, around 8 million people worldwide died from cancer, with an increasing trend, particularly due to the growing population and increasing life expectancy.
For many years, conventional forms of therapy (surgery, radiotherapy and chemotherapy) dominated the treatment of cancer with moderate success. Only in the last decade have alternative and increasingly personalised therapy concepts made significant progress in cancer treatment. Among the great hopes are immunotherapies using immune checkpoint inhibitors or oncolytic viruses (OVs, cancer-killing viruses).
Although the therapeutic potential of viruses has been known for a long time, the FDA approval of the first oncolytic virus Imlygic® in 2015 for the treatment of malignant melanoma marks a breakthrough in tumour therapy.
Oncolytic virus therapy
In general, viruses exploit their host cells to reproduce themselves and most often kill the infected cells. OVs can also lyse and kill tumour cells. However, their mode of action goes far beyond the direct destruction and elimination of tumour cells. OV replication in the tumour microenvironment results in recruitment of immune cells for a local inflammation.
Furthermore, concomitant with tumour cell lysis, tumour-specific antigens are released, thereby eliciting a robust anticancer immune response. This multimodal effect of OVs is expected to result in a kind of systemic anti-tumour vaccination potentially supporting cancer regression.
Genetic engineering of viruses allows for the embedding of various functions into the viral genome that potentiate the natural properties of viruses.
Furthermore, in combination with other tumour therapies, eg chemotherapy or immunotherapy, OVs represent promising therapeutic options to revert tumour-immunosuppression and enhance the efficacy of cancer therapies. The therapeutic potential of a number of viruses, including herpes-, measles, vesicular stomatitis, parvo- and coxsackie viruses, is currently explored in numerous clinical trials worldwide, providing insight into the safety and potency of OVs used in cancer therapy.
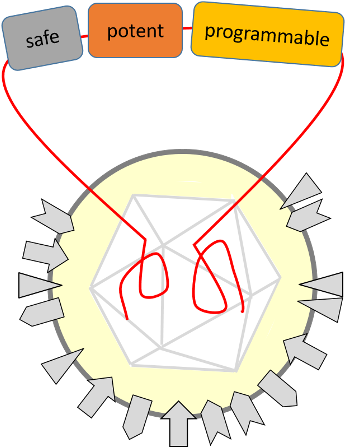
TheraVision: Towards safe, potent and programmable OVs
Results of clinical trials using OVs are promising; however, to be transferred into a widely used option for cancer treatment, several limitations need to be addressed. More potent viruses are needed that are engineered and programmed to specifically replicate in and kill cancer cells but do not affect normal cells. Their ability to activate the immune system, to revert the tumour-immunosuppression and efficiently spread in the tumour microenvironment need to be addressed.
Furthermore, an OV-based vaccine effect is highly desirable for a long-term and systemic tumour eradication. Last but not least, OVs should be further engineered for minimised host defence reactions and side effects.
TheraVision constitutes a complete development pipeline including (i) a proprietary HSV1 engineering vector that facilitates programming of viruses with multiple functions being safe and potent at the same time, (ii) scalable and robust methods for virus production in a GMP-compliant process and (iii) complex preclinical test systems that include human tumour and immune cells to demonstrate sensitivity and safety of combined virus-immunotherapies.
A platform technology for the versatile programming of an oncolytic HSV1
To establish a platform technology for the development of a programmable oncolytic virus, a clinical herpes simplex virus type 1 (HSV1) isolate with high potency and acyclovir sensitivity was selected. The genome of this HSV1 isolate was cloned as a bacterial artificial chromosome (BAC) to make it amenable to simple and targeted manipulation by bacterial genetics. Viruses reconstituted from these BACs were comparable in growth characteristics to the clinical isolate.
Since this platform vector allows for versatile programming of the HSV1 genome by facilitated integration of transgenes, as well as high-capacity engineering, it is highly suited as a research tool. Attenuation of the viral vector was achieved by impairing neurotoxicity genes of HSV1. Infection of a mouse model revealed that compared to an unmodified HSV1 vector, the viral load in the mouse brain was significantly reduced thus demonstrating its safety.
For facilitated functionalisation using high-capacity engineering, specific integration sites were identified in the herpes viral genome. The insertion of transgenes resulted in highly potent functionalised viruses expressing novel protein functions such as immune modulators.
As proof of concept, OVs were developed to target the Non-Small Cell Lung Carcinoma (NSCLC). For optimised targeting to tumour cells and immune modification of the tumour microenvironment, transgenes were implemented into the platform vector. The newly developed OVs efficiently and specifically replicated in tumour cells and virus-encoded immune checkpoint inhibitors proved to be functional by binding the receptors of the immune system.
Thus, transgenes for targeted virus-mediated oncolysis and immune cell-mediated therapy are being combined into a single viral vector, resulting in the efficient destruction of tumours and potentially even metastases.
Development of a viral production process
For the production of HSV1 as active pharmaceutical ingredient, a scalable pharmaceutical bioprocess (API) was developed. A smart combination of already established unit operations with mathematical modelling resulted in a manufacturing process characterised by generic robustness, as well as high yields, biological activity and purity.
The process sequence represents a generic virus production platform applicable to manufacturing of other viral vectors and designed to meet GMP requirements.
Preclinical testing of oncolytic viruses
In parallel, preclinical test systems containing both human tumour and immune cells were established addressing different endpoints: 2D cell lines and 3D organoid models are being established based on a cell and tissue bank from cancer patients, thereby enabling initial efficacy testing in the presence of autologous immune cells.
The pathological microenvironment of the tumour is depicted in complex 3D in vitro tumour models on a biological tissue matrix that for instance allow for testing of different NSCLC cell lines. Complex humanised animal models report on the sensitivity of the OVs using expression of a reporter protein in NSCLC cell lines that enables a highly sensitive bioluminescence detection of the tumour. Using these preclinical test systems, we could show that the engineered OVs enable the specific infection and destruction of the tumour cells and specifically influence the immune system.
Thus, with TheraVision, a broadly applicable platform technology for tailored oncolytic virus- and immunotherapies based on HSV1 has been established. Core competencies to complete the entire production process of this oncolytic virus technology are offered by the contributing institutions.
Literature:
Bailer SM, Funk C, Riedl A, Ruzsics Z. (2017) Herpesviral vectors and their application in oncolytic therapy, vaccination, and gene transfer. Virus Genes 53(5):741-748
Contributing consortium
Funk C1, Seitz C2, Küfer K-H3, Dandekar G4, Uhlig N5, Grunwald T5, Werno C2, Bohle K2, Ziehr, H2, Bailer SM1
1 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB, Stuttgart, Germany; 2 Fraunhofer Institute for Toxicology and Experimental Medicine ITEM, Braunschweig & Regensburg, Germany; 3 Fraunhofer Institute for Industrial Mathematics ITWM, Kaiserslautern, Germany; 4 Fraunhofer Institute for Silicate Research ISC, Würzburg, Germany; 5 Fraunhofer Institute for Cell Therapy and Immunology IZI, Leipzig, Germany.
*Please note: This is a commercial profile
© 2019. This work is licensed under CC-BY-NC-ND.