The REWAISE project is developing electrochemical capture of Li from brines using spent battery electrodes to help alleviate water scarcity
The massive and improper use of natural resources in the last centuries had aimed the world at an extreme climate emergency. Nowadays, 700 million people suffer water scarcity, and the research community alerts that around two-thirds of world population could live under water stress conditions by 2050(1). In addition, the water sector is a major energy consumer, which implies a significant C footprint.
Considering these environmental problems, the REWAISE(2) project (REsilient WAter Innovation for Smart Economy), focused on developing a new “smart water ecosystem” arises.
To address this ambitious aim, a consortium consisting of 25 private and public organisations, led by Aqualia, will work for five years on novel carbon-free and hydrologically sustainable alternatives that meet the targets of water circularity. Thus, reducing the consumption of fresh-water and energy, and recovering water resources, including critical raw materials (CRMs), are the main focuses of REWAISE.
Regarding CRMs, the European Commission has outlined a list that includes several materials due to their economic importance and supply risk. CRMs are highly used in three strategic sectors which are expected to grow in the near future – renewable energy, e-mobility and defence and aerospace.
Many of those activities require the coupling of energy storage systems (ESs). To meet the requirements of a novel “net zero emission” energy system based on renewable energy production, around 9000 GWh of ESs must be installed by 2050(3). The development of Li-ion batteries (LIB) will be key to face up that expected worldwide demand.
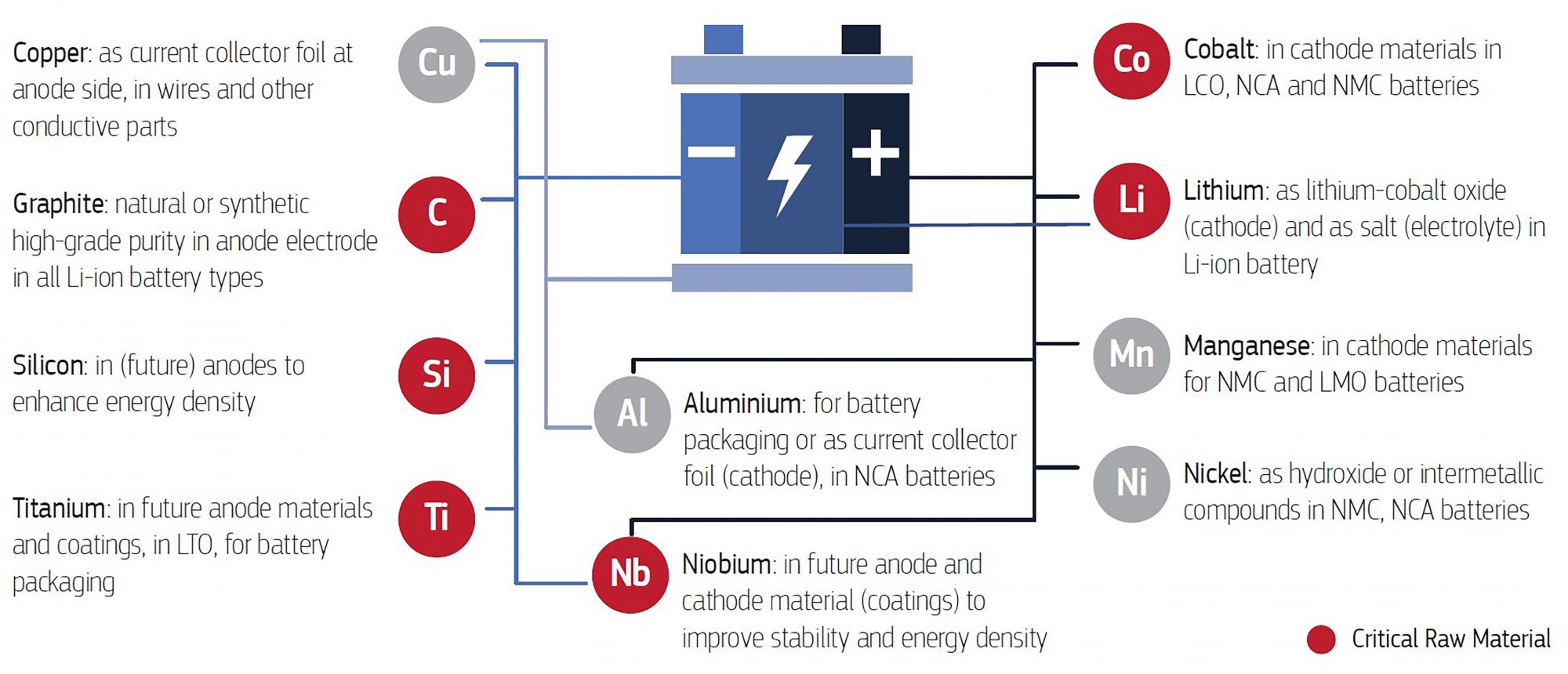
Nevertheless, many of the components of a LIB (Figure 1) are made of CRMs, which point out that the supply of ESs could be jeopardised.
According to that, the recovery of lithium from seawater brines is one of the goals of REWAISE project that is being addressed by CETIM Technological Centre.
Recovery of Li from seawater desalination brines
Considering that only 2.5% of the total water in the Earth is freshwater(5), the desalination of seawater by means of membrane processes has been proposed as one of the best alternatives to produce freshwater. Despite the advantages of these techniques to produce freshwater, a brine stream must also be obtained, bringing out a new residue that has to be managed. Bearing in mind the composition of a brine, its huge concentration of salts turns it into a source full of resources that may contain CRMs as lithium. The concentration of lithium in seawater is around 0.17 mg dm-3 but it can be 2 to >3 times higher in desalination brines. The total amount of Li in seawater reaches 230 Gt.
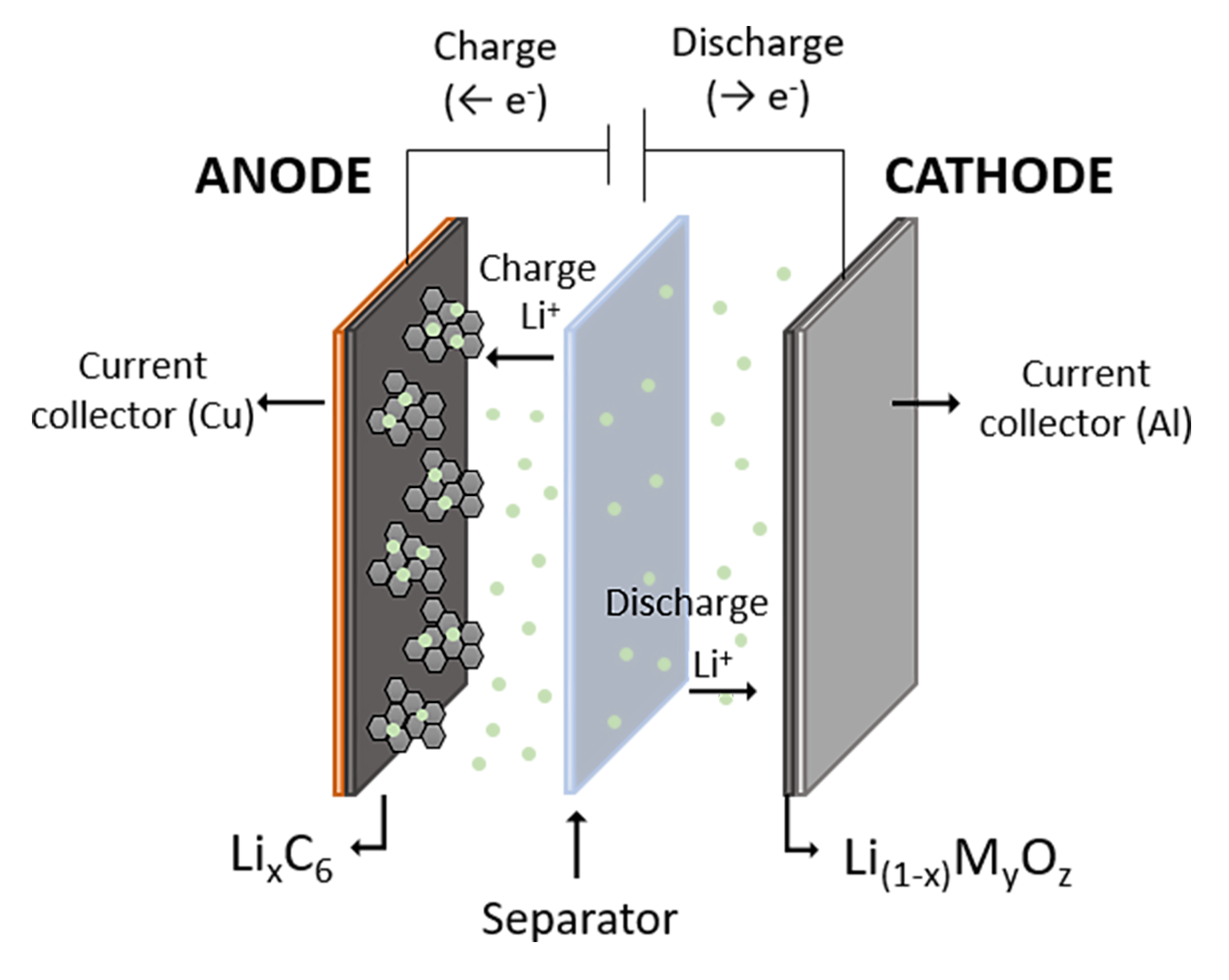
Considering the previous description, CETIM will work on the development of a lithium recovery system based on the electrochemical mechanisms of LIBs (Figure 2). In these batteries, lithium is released from the cathodic surface (LiCoO2) during the charge step. The released lithium-ions travel throughout the separator until the anodic compartment.
Conversely, in the discharge step, the lithium-ions are transported from the anodic chamber to the cathodic one and they are deposited on the cathodic electrode. Following the discharge mechanism of a LIB, the REWAISE project seeks to recover the lithium in brines by means of an electro-deposition technique. This will ensure the selectivity of the recovery process and fulfil the strategies of the circular economy. Cathodic electrodes of spent batteries will be used as an active material of this novel electrochemical resource recovery treatment.
REWAISE progress
Firstly, a deeper physic-chemical characterisation of different cathodic materials has been carried out. Using cathodic electrodes of spent LIB as selective electrode of this recovery process could expose a wide and heterogeneous variety of materials that may show different performances. Consequently, identifying the structure (LiMO2) and the composition of the cathodic material is essential to ensure a reliable accuracy, repetitiveness, and performance of the recovery process.
Once the physic-chemical characteristics of the available cathodic electrodes are known, an exhaustive electrochemical characterisation of those materials shall be performed to determine the influence of the cathodic material and the lithium content on the lithium recovery performance.
These trials will shed light on new and essential insights to achieve the three key components of REWAISE circular water concept: value in, from and through water.
References
(1) United Nations (UN). www.un.org Water scarcity.
(2) REWAISE. www.rewaise.eu
(3) International Renewable Energy Agency (IRENA) Global
Renewables Outlook: Energy transformation 2050 (2020) www.irena.org
(4) European Commission (2020) Critical materials for strategic
technologies and sectors in the EU – a foresight study
(5) National Geographic (2021) Freshwater Crisis. www.nationalgeographic.com

REWAISE | Resilient Water Innovation for Smart Economy has received funding from the European Union’s HORIZON 2020 Research programme under the Grant Agreement no. 869496.
Please note: This is a commercial profile