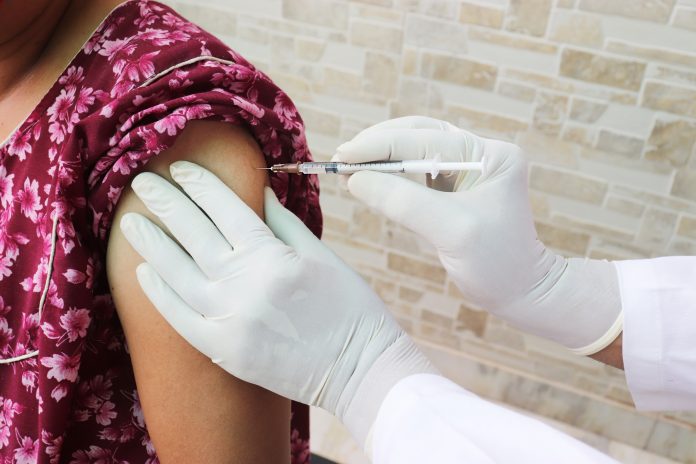
Today (3 November) the World Health Organisation has approved the COVID vaccine, Bharat Biotech Covaxin, for Emergency Use Authorisation
The Bharat Biotech COVID vaccine, Covaxin, appears to be 81% efficient against the virus in Phase Three of clinical trials. Now, it has been approved by the WHO for Emergency Use.
According to the company, they will be able to create 50-55 million doses per month – beginning October, 2021. The vaccine technology used will be shared with manufacturers in India, the US and other countries.
Due to World Trade Organisation (WTO) restrictions via the TRIPS Agreement, the exact recipes of vaccines cannot be currently shared with manufacturers. This means that there is less supply available to buy, while South Africa struggles to maintain public health. Here, we get policy insight on what could be done differently to change this vaccine stasis.
How does the COVAXIN vaccine work?
The Bharat Biotech vaccine uses a dead virus, which triggers the body to create antibodies without needing to insert a living version of COVID-19. This is thought to create fewer side effects, but real world impact data is necessary. The company has been creating vaccines for 24 years, and exporting to 123 countries.
COVAXIN uses a similar vaccine technology to seasonal flu shots. It is currently being used in India’s vaccine rollout, before late stage clinical information was available.
“Successful public-private partnership” says Bharat Biotech Director
Mrs. Suchitra Ella, Joint Managing Director, Bharat Biotech, said: “The WHO nod for COVAXIN® is a validation of the tremendous effort made by everyone at Bharat Biotech and our partners. It is also an opportunity for us to create meaningful impact at a global level. Such an impact can only be orchestrated when multiple stakeholders come together to work towards a common goal. COVAXIN® is a great example of a successful public-private partnership in developing a world-class COVID-19 vaccine.”