Graciela A. Unguez and Chiann-Ling C. Yeh from New Mexico State University explain that sarcomere disassembly is a naturally occurring event during tail regeneration in the adult long-tailed knifefish S. macrurus
Perhaps the most characteristic feature of skeletal muscle invertebrates is its regular pattern of striations, comprised of many hundreds of identical repeated units called sarcomeres1. How so many proteins come together so eloquently to form each of these highly organised myofibrillar structures present in vastly different muscles in very different animal phyla remains a central question in muscle biology. Many (>100) of the protein components that make up a sarcomere invertebrate have been identified2 and the spatial distribution of these proteins within the sarcomeric unit has been well characterised3.
Sarcomere proteins are compartmentalised into functional regions that include the Z-disk, which flanks the sarcomere and anchors thin filament proteins (such as actin) in the I-band. Thin filaments interact with the thick filament proteins (such as the myosin heavy chain, MHC) of the A-band and these myosin structures are crosslinked with the proteins of the M-line (such as myomesin) 2-3.
How these proteins are localised in different regions of the sarcomere and which proteins are necessary for sarcomere integrity are topics of intense investigation with several groups formulating different models by which the spatial and temporal sequence of sarcomere assembly takes place3-5. Although these models diverge in how sarcomeres are formed, they all propose that sarcomere assembly is a well-ordered stepwise process.
In contrast to the efforts on understanding sarcomere formation, relatively few studies have characterised the sarcomere disassembly process – a process that occurs under atrophy conditions and muscle loss following trauma or degenerative disorders such as sarcopenia, muscular dystrophy, or amyotrophic lateral sclerosis6. These studies have largely been under conditions of induced changes in specific sarcomere gene expression using genetic animal models7-10.
Whether sarcomere structures get dismantled in a temporal sequence that is in the exact reverse order of that followed in their assembly remains unknown. Remarkably, sarcomere disassembly occurs in the gymnotiform Sternopygus macrurus as part of the process by which some muscle cells convert into electrocytes, i.e., the non-contractile electrogenic cells of the electric organ, during tail regeneration11. After tail amputation, myogenic precursors in the regeneration blastema give rise to multinucleated muscle cells and some of these myotubes proceed to fuse, disassemble sarcomeres and downregulate many sarcomeric proteins to form electrocytes11.
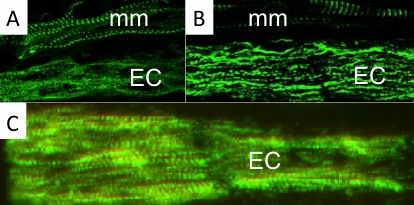
Using a combination of ultrastructural and immunolabeling approaches, we have begun to analyse changes in sarcomere structures and sarcomeric protein expression at different stages of the muscle cell-to-electrocyte conversion within each regeneration blastema two weeks after tail amputation (Fig. 1). These data confirm the proximal-to-distal cell differentiation gradient where regenerating electrocytes most proximal to the tail amputation site are more differentiated (i.e., less sarcomeric structures) than those regenerating electrocytes most distal (more striations) to the amputation site (Fig. 1). Moreover, the breakdown of sarcomeres during the formation of electrocytes was found to occur in a step-wise manner.
This temporal sequence of sarcomere disassembly correlated with the changes detected in protein expression obtained with immunolabeling (Fig. 2). First, Z-disks misaligned to form smaller striated myofibrils (Fig. 1C and 2A) followed by the disappearance of the M-line (Fig 2B). The A-bands with MHC were removed (Fig. 1B and 2C) causing sarcomeres to break into IZI components (Fig. 1A). These I-bands (Fig. 1A and 2B) then dispersed throughout the electrocyte and some remained visible in adult electrocytes.
These data suggest a disassembly of sarcomeres that closely follows the reverse order of the model for sarcomere formation proposed by Sparrow and Schock5. Although it remains premature to conclude the characterisation of the molecular and cellular processes that underlie the striking phenotypic conversion of muscle cells into the highly specialised electrocytes, this work clearly extends the application of S. macrurus to studies relevant to sarcomere stability and homeostasis.
Please note: this is a commercial profile
References
- Huddart H. and Kerkut G.A. (1975). The fine structure of skeletal muscle. In: The comparative structure and function of muscle. Pages 4-48. ISBN: 978-0-08-017845-5. Elsevier Inc.
2. Gautel M. and Djinović-Carugo K. (2016). The sarcomeric cytoskeleton: from molecules to motion. Journal of Experimental Biology 219: 135-145.
3. Ehler E. and Gautel M. 2008. The sarcomere and sarcomerogenesis. Advances in Experimental Medicine and Biology 642: 1–14.
4. Sanger JW, Wang J, Fan Y, White J, Mi-Mi L, Dube DK, Sanger JM, and Pruyne D. (2017). Assembly and Maintenance of Myofibrils in Striated Muscle. Handbook of Experimental Pharmacology. 235:39-75.
5. Sparrow JC, Schöck F. (2009). The initial steps of myofibril assembly: integrins pave the way. Nature Review Molecular Cell Biology. 10:293-8.
6. Burd, N.A., Gorissen, S.H., Loon, L.J.V., 2013. Anabolic Resistance of Muscle Protein Synthesis with Aging. Exercise and Sport Sciences Reviews 41, 169–173.
7. Nishii, K., Morimoto, S., Minakami, R., Miyano, Y., Hashizume, K., Ohta, M., Zhan, D.-Y., Lu, Q.-W., Shibata, Y., 2008. Targeted disruption of the cardiac troponin T gene causes sarcomere disassembly and defects in heartbeat within the early mouse embryo. Developmental Biology 322, 65–73).
8. Peng, J., Raddatz, K., Labeit, S., Granzier, H., Gotthardt, M., 2006. Muscle atrophy in Titin M-line deficient mice. Journal of Muscle Research and Cell Motility 26, 381–388.
9. Perkins, A.D., Ellis, S.J., Asghari, P., Shamsian, A., Moore, E.D., Tanentzapf, G., 2010. Integrin-mediated adhesion maintains sarcomeric integrity. Developmental Biology 338, 15–27.
10. Weinert, S., Bergmann, N., Luo, X., Erdmann, B., Gotthardt, M., 2006. M line–deficient titin causes cardiac lethality through impaired maturation of the sarcomere. The Journal of Cell Biology 173, 559–570.
11. Unguez, G.A., Zakon, H.H., 1998. Phenotypic conversion of distinct muscle fiber populations to electrocytes in a weakly electric fish. Journal of Comparative Neurology 399, 20–34. 12 Holtzer H, Hijikata T, Lin ZX, Zhang ZQ, Holtzer S, Protasi F, Franzini-Armstrong C, Sweeney HL. (1997). Independent assembly of 1.6 microns long bipolar MHC filaments and I-Z-I bodies. Cell Structure Function 22:83-93.
Funding Source: This research supported by NSF CISE/CNS and BIO/IOS INSPIRE Award and Howard Hughes Medical Institute’s 2010 Science Education grant 52006932.
Graciela A. Unguez, PhD
Professor of Biology
New Mexico State University
Tel: +1 (575) 646 7963










