African swine fever is placed under the spotlight here by Prof. Dr. José Manuel Sánchez-Vizcaíno, Full Professor of Animal Health at Universidad Complutense de Madrid
African swine fever (ASF) is today the biggest threat of the global swine industry causing serious sanitary and economic consequences. Nowadays, the disease is present in more than 50 countries on four continents (Africa, Europe, Asia and Oceania) and more than 77% of the world swine population is living in an affected area. Control is based on early diagnosis and the implementation of strict sanitary measures that have not been sufficient until now to stop the disease. ASF is spreading widely worldwide. No commercialise vaccine or effective treatment are available.
The VACDIVA project
The EU-funded VACDIVA project (Gran Agreement No. 862874) has been financed with 10 million euros by the European Union (EU) to solve the ASF problem in Europe and other affected countries, providing a safe and effective vaccine(s) for wild boar and domestic pigs ready for registration. It will also develop DIVA (Differentiating Infected from Vaccinated Animals) test to allow accurate monitoring of the effectiveness of the vaccine. Finally, it will design control and eradication strategies in different epidemiological scenarios worldwide and test the pilot vaccine in real environments.
Partners in the project hail from Europe and beyond. VACDIVA counts with the expertise of two world ASF Reference Laboratories (OIE and FAO), the EU ASF reference laboratory (EURL), six EU ASF National Reference Laboratories (of six out of the 10 countries currently affected by ASF) and four prestigious ASF research Institutions. In addition, four non-EU institutes are involved, including ILRI in Kenya, the Chinese institutes IAS-CAAS, as well as Russia’s ARRIAH. It is important to highlight the active involvement of pig producers, hunters and agricultural associations. Two companies are participating in the project: MSD Animal Health, the leader in vaccine products, and INGENASA as a diagnostics company.
The VACDIVA project is focusing on the study and validation of three different vaccine prototypes for Safety and Efficacy in Domestic pigs (DP) and wild boar (WB). The candidate Lv17/WB/Rie1 is a naturally attenuated and non-haemodsorbing genotype II African swine fever virus (ASFV) isolated from a hunted wild boar in Latvia in 2017. The preliminary results with this prototype in DP described the effective dose and route of immunisation, with 100% protection against the virulent challenge without clinical signs, cross-protection and duration of the immunity. In WB conferred 92% protection against heterologous challenge with a virulent genotype II ASFV isolate. This candidate poses the first report of a promising vaccine against ASF in wild boar by oral administration. Therefore, the project is developing different types of oral baits suitable to wild boar administration systems.
The second vaccine prototype, NH/P68, is a naturally attenuated and non-haemodsorbing genotype I ASFV isolated from an infected domestic pig in Portugal in 1968. This candidate showed to confer protection against ASF genotype II and I. This prototype has been already adapted to grow in an established cell line (accepted by EMA regulation for vaccine production) and is then evaluated in DP for the efficacy and cross-protection capacity. The optimal doses and administration route are described to confer 100% protection in DP against heterologous ASF virulent genotype II isolate with a significant reduction of side effect, in comparison to NH/P68 prototype using the NH/P68. In WB, it is already under evaluation to optimise the doses of immunisation and the cross-protection against different ASF circulating isolates.
The third prototype, ASF/ARRIAH/CV-1, is an attenuated strain obtained by cell culture passage in Vladimir Laboratory. In contrast with the previous ones, the in vivo protection has not been tested yet.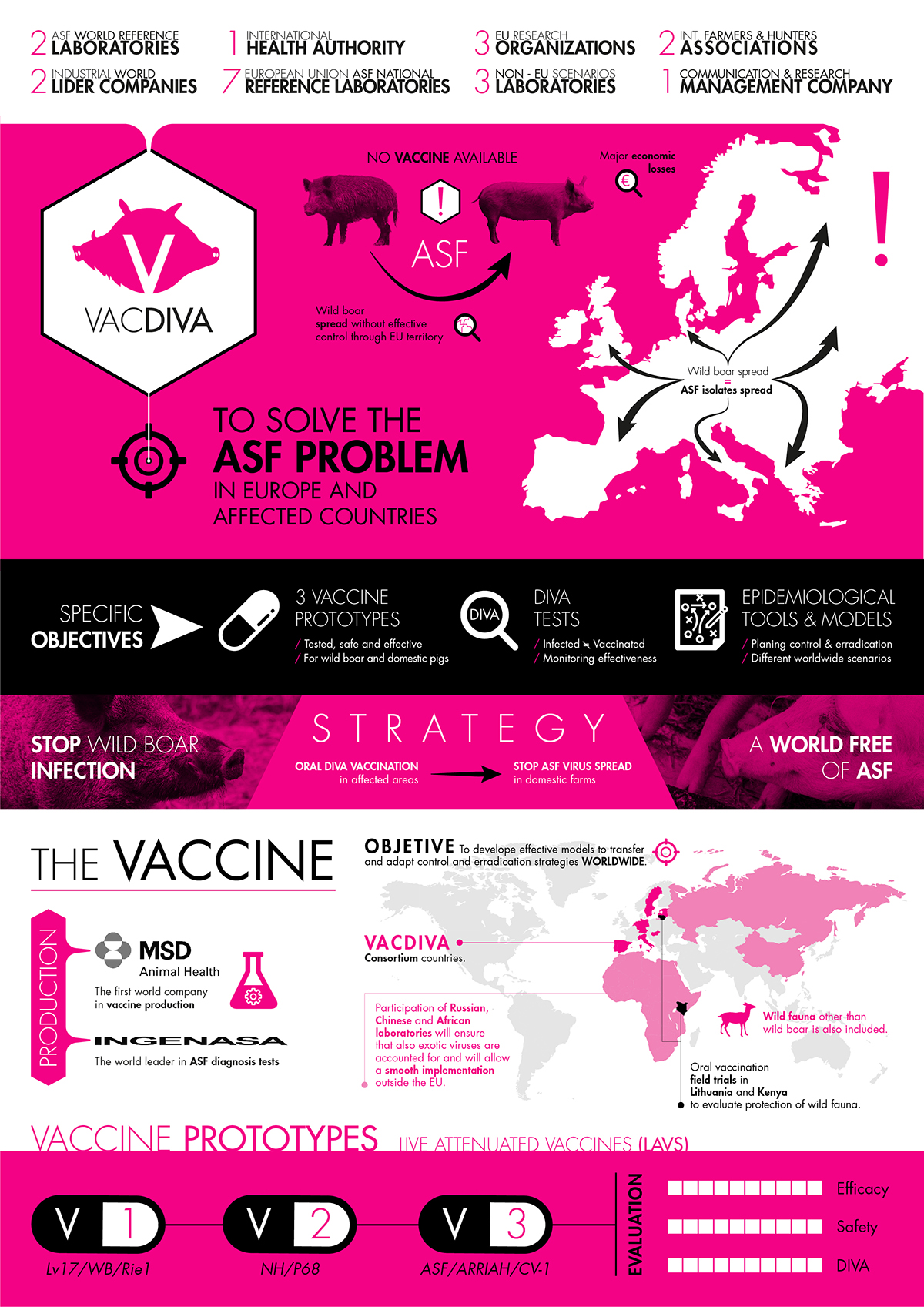 Two proteins from the first prototype have been described for the DIVA test as well as one for the second prototype. That will allow differentiating vaccinated from infected animals. The project has also produced different deletion mutants from the first prototype that are ready for the in vivo evaluation in DP. The partners involved are working to obtain others from the second prototype.
Two proteins from the first prototype have been described for the DIVA test as well as one for the second prototype. That will allow differentiating vaccinated from infected animals. The project has also produced different deletion mutants from the first prototype that are ready for the in vivo evaluation in DP. The partners involved are working to obtain others from the second prototype.
The project is also studying vaccine manufacturing. Since pig blood cells are not an option, few candidates of a cell line are being already selected.
The ultimate goal of this project is to generate safer and efficacy vaccines with DIVA properties and a control and eradication strategy for the vaccination in different ASF scenarios.
Keep up-to-date with the VACDIVA Project
Stay up to date with the VACDIVA project’s latest news and progress and visit our website: www.vacdiva.eu. Among others, you will find the African Swine Fever section with the most important news and information about the disease (videos, ASF outbreak interactive map, latest news). Also, you will be able to follow the VACDIVA Work packages progress and keep updated on all the activities (meetings, workshops, seminars, publications) performed by the project.
Don’t forget to subscribe to our annual e-newsletter.
For questions and requests, please use the contact form: https://vacdiva.eu/contact/
VACDIVA, GA has received funding from the European Union’s HORIZON 2020 Research programme under the Grant Agreement no. 862874.
Please note: This is a commercial profile
© 2019. This work is licensed under CC-BY-NC-ND.