Contributing scientists share their thoughts around the field of chemistry, focussing on enzyme activity assays for disease diagnosis, novel drug development validation and nanoparticles
Shu Jia, Jay Sibbitts, Abby Kreznor, Dr Obdulia Covarrubias-Zambrano, Jose Covarrubias, Dr Madumali Kalubowilage, Stefan Bossmann, and Christopher Culbertson from Kansas State University
Enzyme activity assays can be used for disease diagnosis and novel drug development validation. Understanding how well a key enzyme functions in a physiological process often reveals early signs of disease that has a long latency period.
Matrix metalloproteinases (MMPs) are a family of matrix-degrading enzymes broadly expressed in normal and pathological processes. Structural proteins, such as collagens, are major targets of MMPs. For example, wound healing involves blood vessel epithelial cell reorganisation which requires the degradation of the original matrix and formation of a new matrix. Upregulation of MMP activities is also commonly observed in cancer metastasis where inter-cell adhesion proteins are decomposed to facilitate tumour cell migration. Measuring MMP activities provides information on cancer development. (1)
Synthetic peptide substrates are useful tools for MMP activity assays. A peptide substrate is designed to include consensus sequences that are recognised and cleaved by a specific MMP enzyme. The cleavage reaction rate is MMP activity-dependent and is determined via measuring the hydrolyzed product or unhydrolyzed substrate (or both). Though the concept is simple, there is one major limitation caused by the way our body controls MMP activity: these enzymes are strongly regulated in the matrix. Therefore, MMP activities need to be, ideally, measured in the original sample matrix.
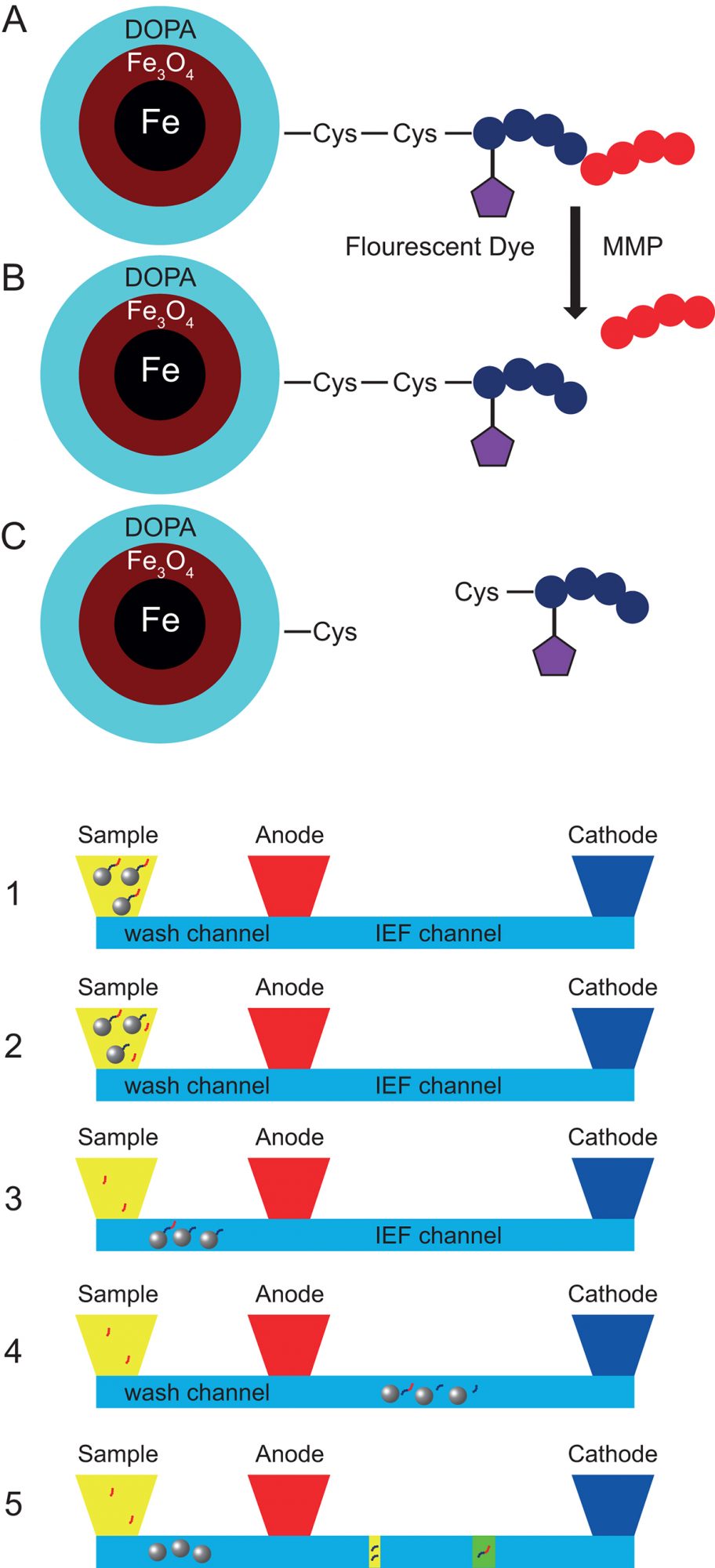
Fig 2. MMP activity measurement processes
Here, we report a novel integrated microfluidic device that can automatically determine enzyme activities that uses peptide substrates attached to a nanoparticle platform and isoelectric focusing (IEF) separation of the substrates after reaction with the enzymes. Magnetic nanoparticles used in this device can be easily dispersed and retrieved from liquid samples, such as blood. (Fig 1.). Cleavable peptide substrates are bonded to magnetic nanoparticles via a disulfide bond which is breakable in a reducing environment. The peptide is labelled with a fluorescent dye for the convenient optical detection. Peptide substrates are cleaved when exposed to MMP enzymes, the reaction rate of which depends on sample MMP activities.
The workflow of the microfluidic device is illustrated (Fig 2.). Liquid samples such as blood or tissue fluids are collected for immediate analysis or storage at -20°C. Approximately 30 μL of sample is added to the sample reservoir where the sample and the synthesised magnetic nanoparticles are mixed (Fig2-1). With a Neodymium magnet underneath the microchip, the nanoparticles are vortex mixed with the sample. After 30 minutes incubation, a portion of the substrates on nanoparticles have been digested by the MMP enzyme (Fig 2-2). The undigested substrates and partially cleaved substrates both remain on the nanoparticles. All of the nanoparticles are transported from the sample reservoir into the wash channel (Fig 2-3). The wash channel contains physiological buffer that rinses off any MMP enzymes adsorbed on the nanoparticle surface to prevent further reaction and provides a clean sample for IEF. The nanoparticles are then transported into the IEF separation channel (Fig 2-4). The IEF separation channel contains reducing reagents that break the disulfide bonds connecting the substrates to the nanoparticle surface. After the detachment of substrates, magnetic nanoparticles are removed from the IEF separation channel. Digested and undigested substrates show different isoelectric points and are separated into two narrow zones in the separation channel (Fig 2-5). A laser-induced-fluorescence detector scans the IEF channel and collects the fluorescence readout from the two focused peptide substrates.
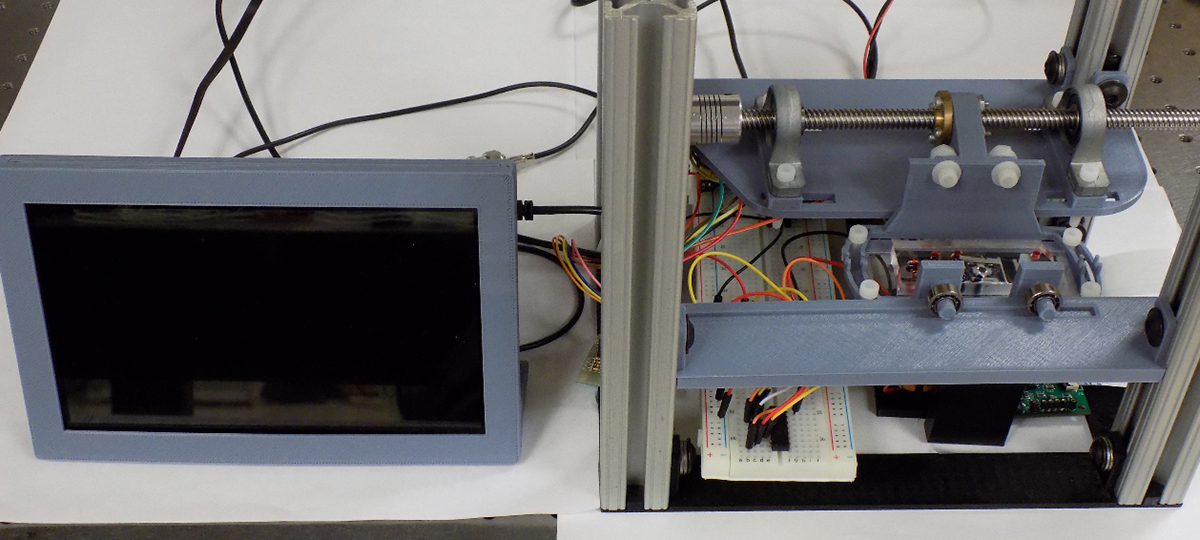
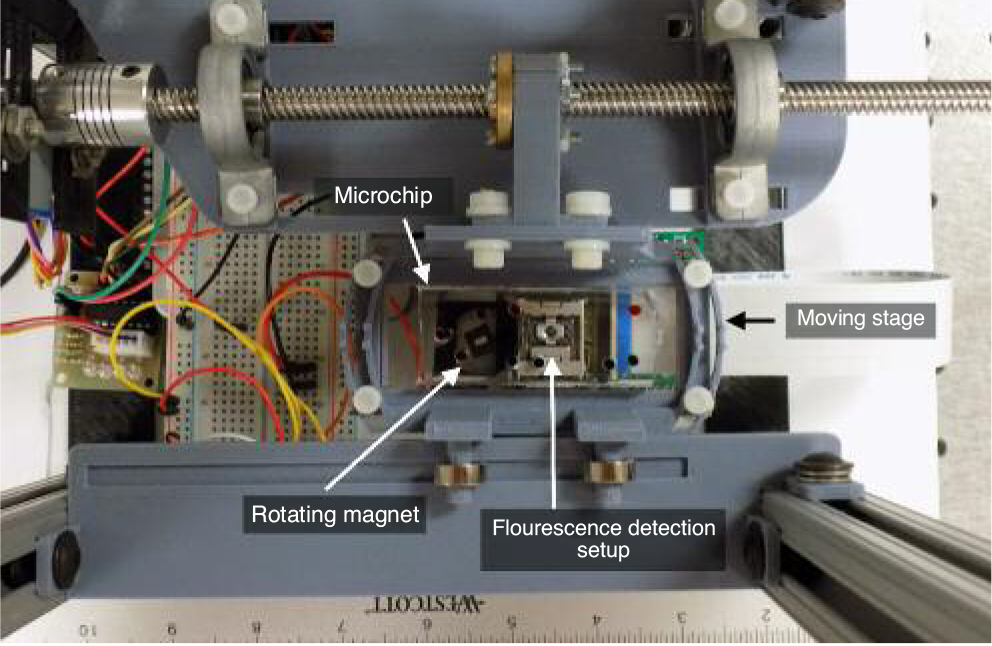
This microfluidic device has been integrated into a sample-in answer-out style workstation. (Fig 3.) The workstation consists of a minicomputer, a linear scanning moving stage, a stationary laser-induced fluorescence detector, a motorised magnet for on-chip nanoparticle manipulation and the microfluidic chip. (Fig 4.)
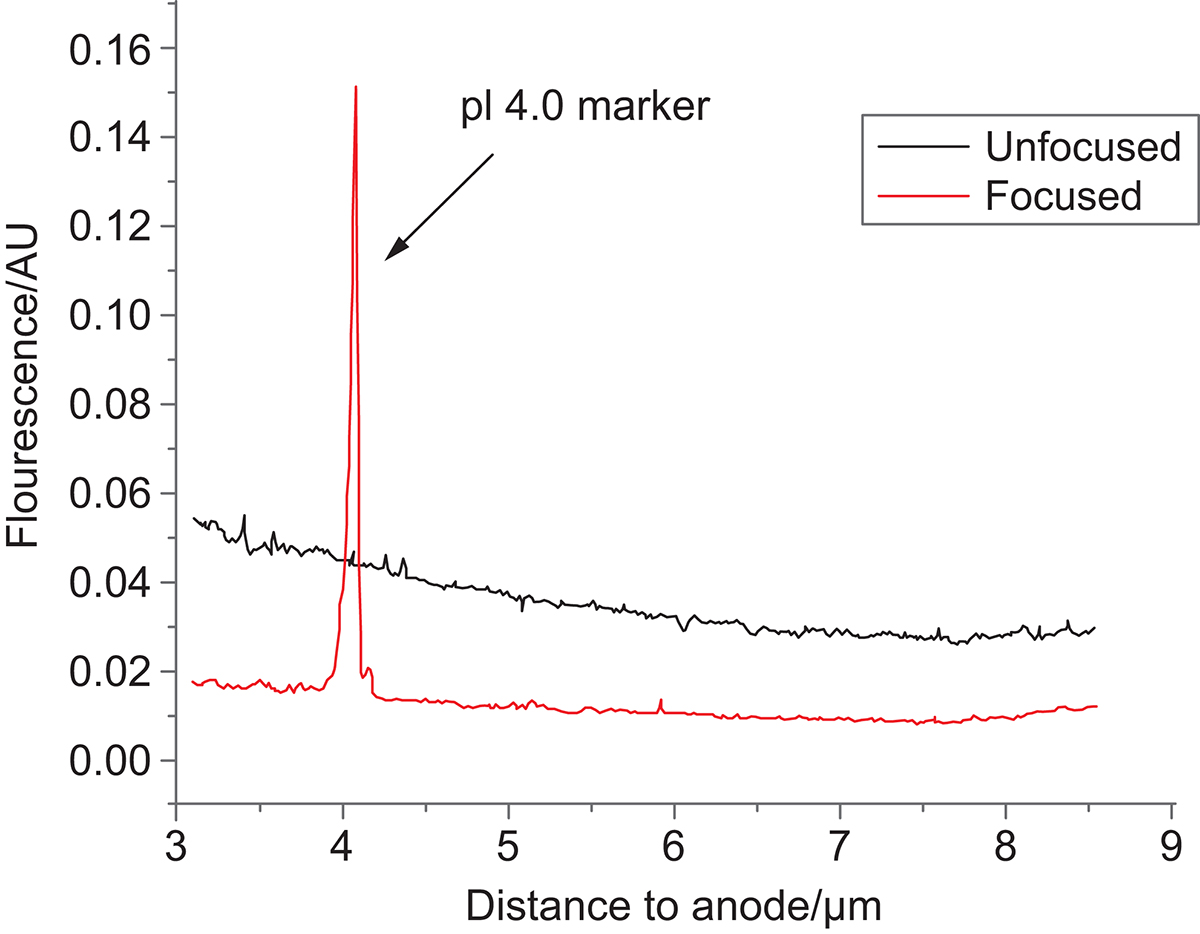
An example of isoelectric point 4.5 peptide standard is shown. (Fig 5.) Under a pH gradient 3-10, the peptide standard is focused close to the low pH end (anode). This also demonstrates that peptides can be designed with an expected isoelectric point. This opens up possibilities to use multiple MMP substrates simultaneously with each substrate designed to be focused at different isoelectric points.
References
Kalubowilage, M.; Covarrubias-Zambrano, O.; Malalasekera, A. P.; Wendel, S. O.; Wang, H.; Yapa, A. S.; Chlebanowski, L.; Toledo, Y.; Ortega, R.; Janik, K. E.; Shrestha, T. B.; Culbertson, C. T.; Kasi, A.; Williamson, S.; Troyer, D. L.; Bossmann, S. H., Early detection of pancreatic cancers in liquid biopsies by ultrasensitive fluorescence nanobiosensors. Nanomedicine (N. Y., NY, U. S.) 2018, 14 (6), 1823-1832.
Please note: This is a commercial profile