Here, Katharine Moore Tibbetts explores laser ablation in liquid as a versatile synthetic technique
Laser ablation in liquid (LAL) is a versatile synthetic technique that rapidly produces nanoparticles from simple precursor materials by focusing an intense laser beam into a liquid or onto a solid-liquid interface. LAL protocols have numerous advantages over traditional wet-chemical methods used to prepare colloidal nanoparticles and nanocomposite materials. LAL produces useful quantities of nanoparticle products with only minutes of laser processing and little post-synthetic workup, as compared to the hours or days typically required with traditional methods.
No toxic reducing agents or organic stabilizers are needed with LAL, resulting in minimal waste generation and making LAL a “green” synthetic method. The lack of organic surfactants for stabilisation also results in “naked” nanoparticle products that are highly desirable for catalytic and biomedical applications. The highly non-equilibrium environment in the laser plasma can freeze metastable material phases, generating novel material structures to advance nanotechnology applications. For these reasons, LAL research has blossomed over the last decade and LAL-synthesised nanomaterials are increasingly being used in industrial applications1.
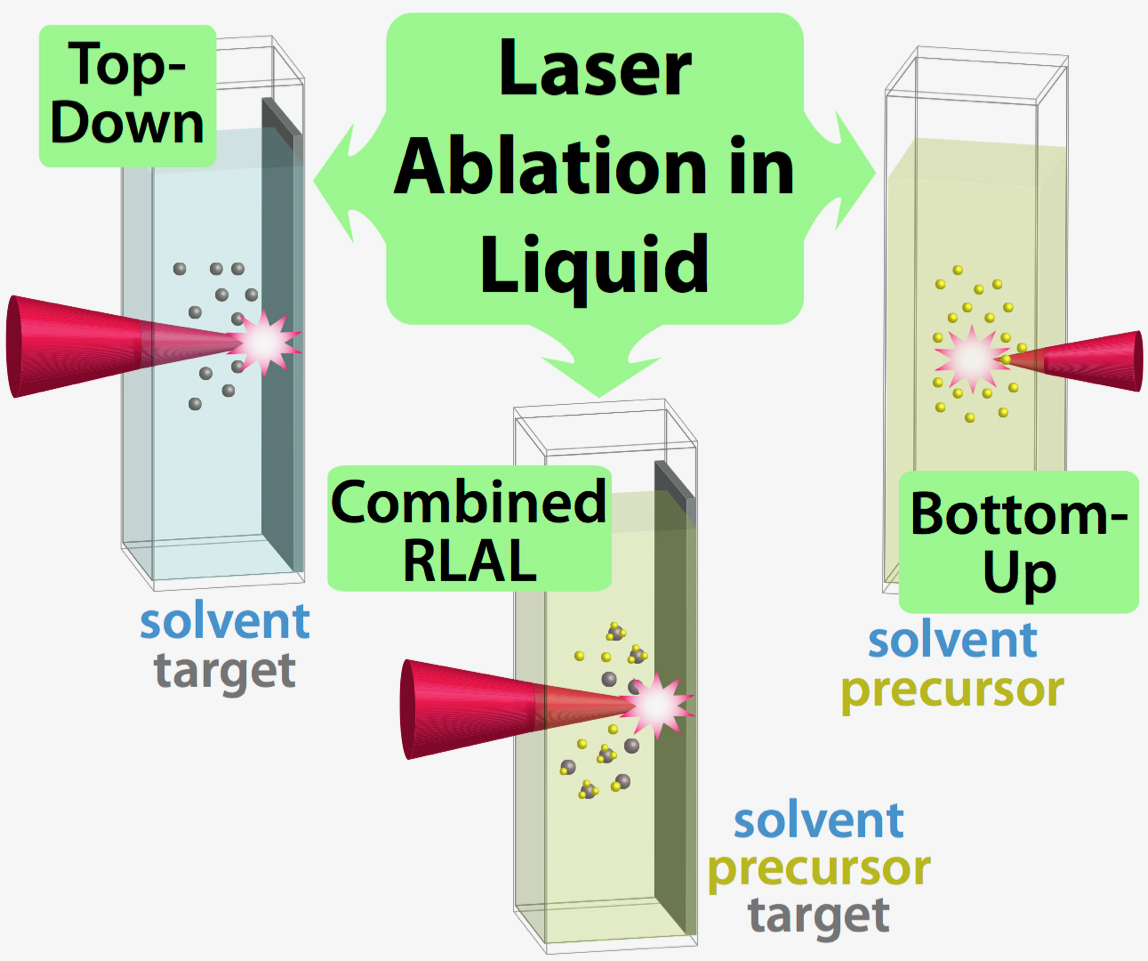
LAL comprises three general approaches, illustrated in Figure 1: top-down, in which nanoparticles are produced from material blasted off a solid target; bottom-up, in which nanoparticles are nucleated and grown from a molecular precursor; and the combination of these, called reactive laser ablation in liquid (RLAL). In all of these approaches, the interaction of focused high-power pulsed lasers with condensed-phase media produces a dense plasma containing electrons, ions, and radicals, along with associated transient temperatures exceeding 5000 K.
The rapid quenching of this plasma by the surrounding liquid arrests the growth of atomic and cluster species formed in the plasma, leading to nanoparticle formation. The complex liquid environment in RLAL provides a unique means to combine multiple elements into a single nanostructured product from the plasma reactions of precursor and solvent molecules with atoms and clusters ablated off of the solid target. Whereas top-down ablation of a metal target in water will produce nanoparticles of that metal and/or its oxides, RLAL of a metal target in metal salt solution can produce metal (oxide) alloys, complex minerals, and metal clusters on oxide supports.
Our laboratory has primarily focused on understanding the chemical reactions that convert metal salt precursors to nanoparticles in bottom-up LAL, focusing on how electrons and hydroxyl radicals contribute to nucleation and growth of gold nanoparticles from aqueous tetrachloroaurate2. From this knowledge, we were able to develop methods for preventing detrimental effects of hydroxyl radicals on nanoparticle properties by adding chemical scavengers to remove them3,4.
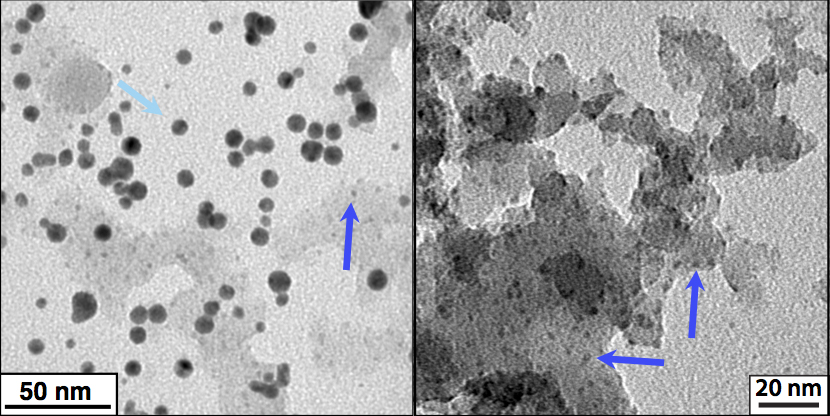
Recently, we have turned to applying these lessons in controlling LAL chemistry to RLAL of a silicon wafer immersed in metal salt solution, with the goal of producing metal-silica nanocomposites. Silica is a popular support to stabilize metal nanoparticles due to its chemical inertness and high thermal stability. We began with a silicon wafer immersed in aqueous tetrachloroaurate salt, as used in our earlier experiments. This RLAL synthesis resulted in two different products, as shown in Figure 25.
Large ~7 nm isolated gold nanoparticles were found to co-exist with a composite product containing much smaller ~2 nm gold clusters supported on an amorphous silica matrix. We rationalised the formation of isolated gold nanoparticle byproduct based on the facile and rapid reduction of tetrachloroaurate to gold nanoparticles in bottom-up LAL as reported in our earlier work2-4. Despite the formation of this undesired byproduct, the gold-silica composite material could easily be separated with centrifugation for further use.
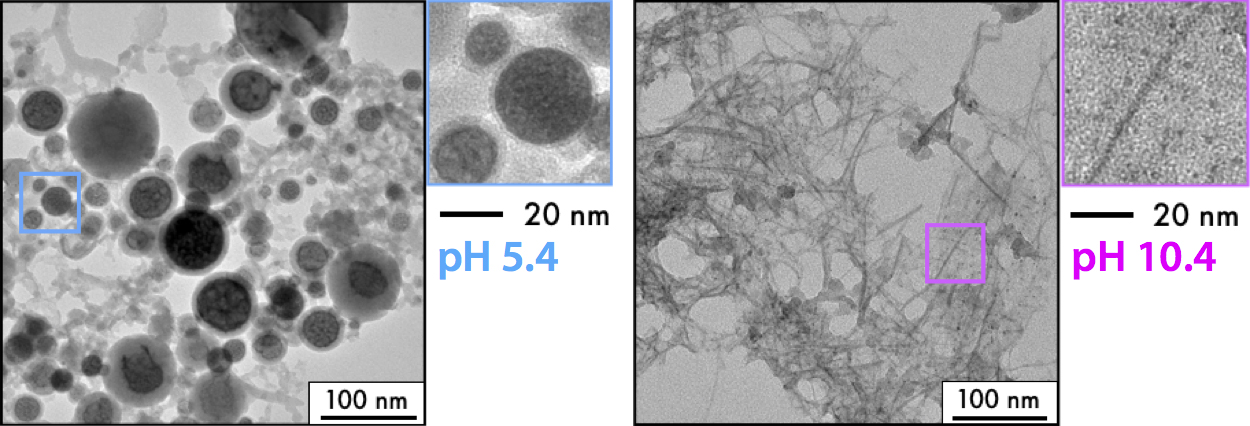
Building on this experience with an easily reducible metal, we decided to investigate RLAL with a copper salt precursor. Unlike gold, salts from metals such as copper cannot be converted to nanoparticles in bottom-up LAL due to their rapid back-oxidation by the hydroxyl radicals produced in the laser plasma, even in the presence of radical scavengers. As a result, RLAL of a silicon wafer immersed in copper(II) nitrate solution formed very different products as compared to gold6. Figure 3 shows the products synthesised at pH 5.4 (a) and 10.4 (b). While acidic conditions produce large core-shell structures, sub-2 nm copper clusters and unusual ribbon-like morphologies attributed to copper phyllosilicate are formed under basic conditions.
These results highlight the potential for RLAL to produce diverse composite nanostructures with morphologies, compositions, and phases unattainable by traditional synthetic methods. Our laboratory’s investigations into the fundamental chemical reaction mechanisms occurring in RLAL will ultimately equip the LAL community with the knowledge needed to synthesise complex nanocomposites with tailored properties for specific applications.
References
1 D. Amans, W. Cai, and S. Barcikowski, Appl. Surf. Sci. 488 445-454 (2019)
2 V. K. Meader, M. G. John, C. J. Rodrigues, and K. M. Tibbetts, J. Phys. Chem. A 121 6742-6754
3 V. K. Meader, M. G. John, L. M. Frias Batista, S. Ahsan, and K. M. Tibbetts, Molecules 23
4 L. M. Frias Batista, V. K. Meader, K. Romero, K. Kunzler, F. Kabir, A. Bullock, and K. M. Tibbetts, J. Phys. Chem. B 123 7204-7213 (2019)
5 M. G. John and K. M. Tibbetts, Appl. Surf. Sci. 475 1048-1057 (2019)
6 In press
*Please note: This is a commercial profile