Professor Jatinder Lamba from the Department of Pharmacotherapy and Translational Research explores the current progress in Acute Myeloid Leukaemia (AML)
Acute Myeloid Leukaemia (AML) is the most common leukaemia in adults and second most common leukaemia in children. AML is very heterogeneous disease characterised by several genomic alterations and cytogenetic abnormalities that are utilised for risk group stratification. Advances in recent years have also identified several mutations of significant prognostic value that are currently being evaluated.
However, in spite of all the efforts to establish the molecular and genetic landscape of AML, improvement in outcome has been dismal. Although initial response to induction therapy is observed in many AML patients’ refractory disease and occurrence of relapse is a major challenge in the successful treatment of AML. The overall 5-year survival for AML ranges from 60% in children to <25% in elderly AML patients with conventional chemotherapy and hematopoietic stem cell transplants, thus warranting the need for newer therapies or redesigning the treatment regimens guided by patient’s genome.
Immunotherapy in AML: An emerging approach to treat AML involves the use of monoclonal antibodies or antibody-drug conjugates. The cell surface antigen currently most exploited is CD33, as it is expressed on AML blasts of the majority of patients as well as on leukemic stem cells making it an attractive therapeutic target. The recognition that antibodies were internalised after binding to CD33 led to the development of an immunoconjugate between an anti-CD33 antibody and a toxic calicheamicin-γ derivative termed Gemtuzumab Ozagamicin (GO Mylotarg™). GO is the first cancer immuno-conjugate to be approved for treatment of CD33 positive AML. GO received an accelerated approval in 2000 for treatment of relapsed AML in older patients(1) but was withdrawn from the market, due to lack of benefit and increased mortality in the FDA-mandated confirmatory trial(2).
However, several follow-up randomised studies demonstrated GO improved outcomes in adult AML when GO was added to initial intensive chemotherapy(2-7); these findings were most pronounced in patients with favourable-risk cytogenetics(8, 9). Within paediatric AML, Children’s Oncology Group trials COG AAML03P1 and COG AAML0531 demonstrated that GO was tolerable(10) and improved event-free survival (EFS), by reducing relapse risk(11).
The data from these trials also suggest that although we see improvement in outcome, the response is very variable suggesting an unmet need for biomarkers predictive of response.
In some of the clinical trials, wide inter-patient variation in CD33 cell surface expression has been reported and benefit from adding GO is observed for those patients with higher CD33 expression (12).
Biomarker predicting GO response
While a lot of emphases is being given in characterisation of a tumour (in this case Leukaemia cell), genome, germline genetic variation (pharmacogenomics) can have a significant impact on drug response. Dr Lamba’s lab focuses on identification, characterisation and functional validation of genomic/epigenomic/pharmacogenomics features predictive of treatment response in AML. Integration of these genomic features with tumour genome can enhance our selection of right agents and prediction of treatment response.
In her quest to identify biomarkers that could presumably predict clinical response to this new and re-emerging drug in AML, her lab investigated whether genetic variation in CD33 gene itself exists in the population and if so whether it impacts clinical efficacy to GO.
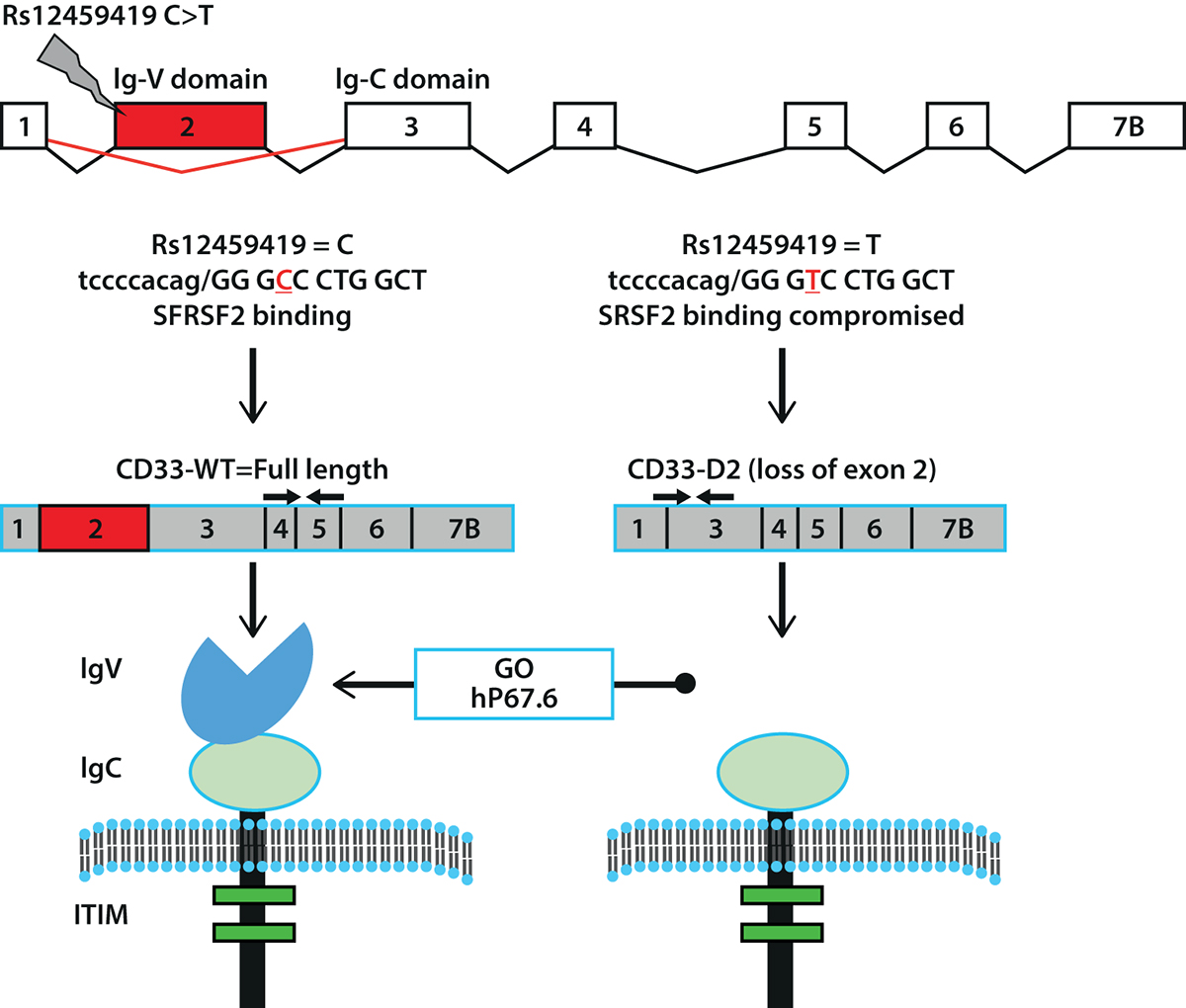
Interestingly Dr Lamba’s group identified several coding single nucleotide polymorphisms (SNPs) in CD33 that were promising(13, 14). Most significant and impressive results so far are the discovery of a splicing and coding SNP in CD33 rs12459419 (C>T; Ala14Val), which is present in exon-2 and alters splicing, resulting in loss of exon-2. Exon-2 codes for IgV-domain and IgV-domain is recognised by both GO and hP67.6-CD33 antibody, which is used for diagnostic immunophenotyping (figure 1).
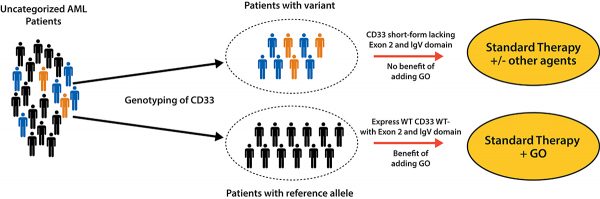
Thus, loss of this domain can not only interfere with the detection of total CD33 but can also compromise therapeutic efficacy of GO by influencing the binding or internalisation of CD33-GO complex. A Recent report from Dr Lamba’s group from the largest-randomised paediatric AML trial ever-AAML0531 demonstrates exactly this point: CD33 splicing SNP is a strong predictor of leukemic cell surface CD33 levels as well as clinical response to GO. Patients with the variant T-allele (CT and TT genotypes) that results in skipping of exon 2 show no difference in outcome between patients treated with or without the addition of GO.
Whereas for patients homozygous for reference allele (CC-genotype) that codes for full-length CD33, a dramatic reduction in relapse risk (from 49% to 26% in NO-GO vs. GO arms) and significant improvement in disease-free survival when given GO (Journal of Clinical Oncology; Lamba et al, 2017) (15).
This is an incredible finding and presence of this variant defines a genotypic driven prediction of response to GO, at a much greater level as compared any of the factors known so far including measurement of CD33 expression levels. Given the recent news from USFDA reapproving GO for treatment of CD33 + AML, once validated, pre-emptive genotype testing of the CD33 variant will set a stage to personalise GO therapy to achieve maximum therapeutic benefit.
Based on the results of the genotype testing, patients likely to benefit from anti-CD33 therapy, such as GO could be selected and at the same time for patients with variant genotype who are less likely to respond to GO-based chemotherapy alternate treatment strategies could be developed (Figure 2). These results could also serve as a significant biomarker to be tested in AML patients for selection of other CD33-based chemotherapy.
These results also bring forward a significant role of pharmacogenomics especially indicating not to ignore host genetic features in our quest to leverage tumour genetics, as variation in host genetics can have a significant impact on response.
This also sets a precedent of probably digging deeper to identify predictive biomarkers in this new era where immunotherapy is being actively pursued in the treatment of cancer.
The research in Dr Lamba’s Lab is funded through NIH grants R01-CA132946 and R21-CA155524

References
- Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukaemia. Clinical cancer research: an official journal of the American Association for Cancer Research. 2001;7(6):1490-6.
- Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J, et al. A phase 3 study of gemtuzumab ozogamicin during induction and post-consolidation therapy in younger patients with acute myeloid leukaemia. Blood. 2013;121(24):4854-60.
- Burnett AK, Hills RK, Hunter AE, Milligan D, Kell WJ, Wheatley K, et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results of the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2013;27(1):75-81.
- Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, et al. Identification of patients with acute myeloblastic leukaemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(4):369-77.
- Burnett AK, Russell NH, Hills RK, Kell J, Freeman S, Kjeldsen L, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukaemia. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(32):3924-31.
- Castaigne S, Pautas C, Terre C, Raffoux E, Bordessoule D, Bastie JN, et al. Effect of gemtuzumab ozogamicin on the survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012.
- Thol F, Schlenk RF. Gemtuzumab ozogamicin in acute myeloid leukaemia revisited. Expert opinion on biological therapy. 2014;14(8):1185-95.
- Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. The lancet oncology. 2014;15(9):986-96.
- Loke J, Khan JN, Wilson JS, Craddock C, Wheatley K. Mylotarg has potent anti-leukemic effect: a systematic review and meta-analysis of anti-CD33 antibody treatment in acute myeloid leukaemia. Annals of haematology. 2015;94(3):361-73.
- Cooper TM, Franklin J, Gerbing RB, Alonzo TA, Hurwitz C, Raimondi SC, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukaemia: a report from the Children’s Oncology Group. Cancer. 2012;118(3):761-9.
- Gamis AS, Alonzo TA, Meshinchi S, Sung L, Gerbing RB, Raimondi SC, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukaemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(27):3021-32.
- Pollard JA, Loken M, Gerbing RB, Raimondi SC, Hirsch BA, Aplenc R, et al. CD33 Expression and Its Association With Gemtuzumab Ozogamicin Response: Results From the Randomized Phase III Children’s Oncology Group Trial AAML0531. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(7):747-55.
- Mortland L, Alonzo TA, Walter RB, Gerbing RB, Mitra AK, Pollard JA, et al. Clinical significance of CD33 nonsynonymous single-nucleotide polymorphisms in pediatric patients with acute myeloid leukaemia treated with gemtuzumab-ozogamicin-containing chemotherapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19(6):1620-7.
- Lamba JK, Pounds S, Cao X, Downing JR, Campana D, Ribeiro RC, et al. Coding polymorphisms in CD33 and response to gemtuzumab ozogamicin in pediatric patients with AML: a pilot study. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2009;23(2):402-4.
- Lamba JKLC, L.; Shin, M.; Loken, M. R. L.; Pollard, J. A.; Wang, Y-C.; Ries, R.; Aplenc, R.; Hirsch, B.; Walter, R. B.; Bernstein, I. D.; Gamis, A. S.; Alonzo, T. A.; Meshinchi, S. CD33 Splicing polymorphism determines gemtuzumab ozogamicin response in de novo AML: report from randomized phase III Children’s Oncology Group Trial AAML0531 Journal of Clinical Oncology. 2017; 10;35(23):2674-2682.
Please note: this is a commercial profile
Professor Jatinder Lamba
Department of Pharmacotherapy and Translational Research
Tel: +1 352 273 6425