Gregory H Hipskind MD PhD and Jody L Mittiga DNM NP from Brain Injury Consulting LLC, reveal their thoughts on evaluating and treating mild traumatic brain injury (mTBI), including the role of SPECT and photobiomodulation therapy
The documented incidence of traumatic brain injury (TBI) has gradually increased in the United States over the last several decades to 2.8 million/year.(1) Published data indicates that approximately 80% of all TBIs fall into the mild category (mTBI) and 10% of individuals sustaining a mTBI may develop permanent neurological sequelae.(2) Given the incidence of newly documented cases of TBI per year, 10% of 2.24 million people (224,000 new people each year) will go on to develop permanent, often disabling symptoms.(3) Additionally, while there are approximately 2.24 million documented cases of mTBI per year, studies estimate the number of undocumented mTBI cases may be much larger.(4) It is possible that as many as 500,000 new cases of permanent brain injury from mTBI may occur in the US annually.
Following mTBI, individuals often experience symptoms of post-concussive syndrome (PCS). PCS symptoms include headache, dizziness, sleep disturbance, personality change, memory problems, attention and affective disturbances. 90% of mTBI PCS symptoms resolve completely. The 10% or so of patients with mTBI whose symptoms do not resolve within three to six months, develop persistent post-concussive syndrome (PPCS). (Fig 1)
TBI as disease process
Recent scientific studies demonstrate that mTBI initiates a neuropathophysiological disease process. (5,6) Elements of this disease process include injury to blood vessels and neurons and initiation of a neurometabolic cascade: influxes of calcium into the neuron affecting mitochondria, alterations in regional cerebral blood flow (rCBF), the sodium/potassium pump, increased neuronal excitation, neuroinflammatory markers and reactive oxygen species.
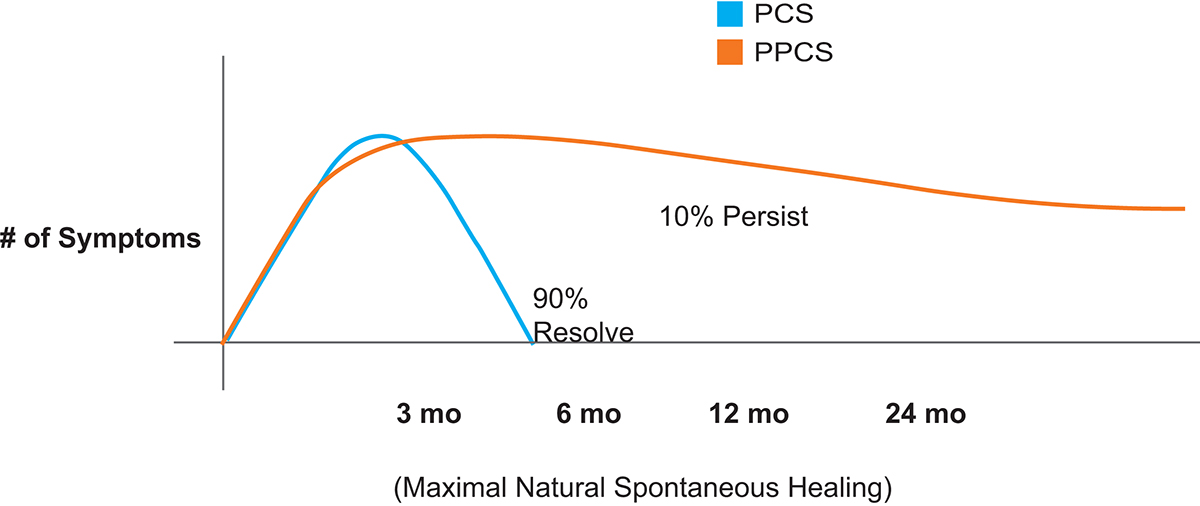
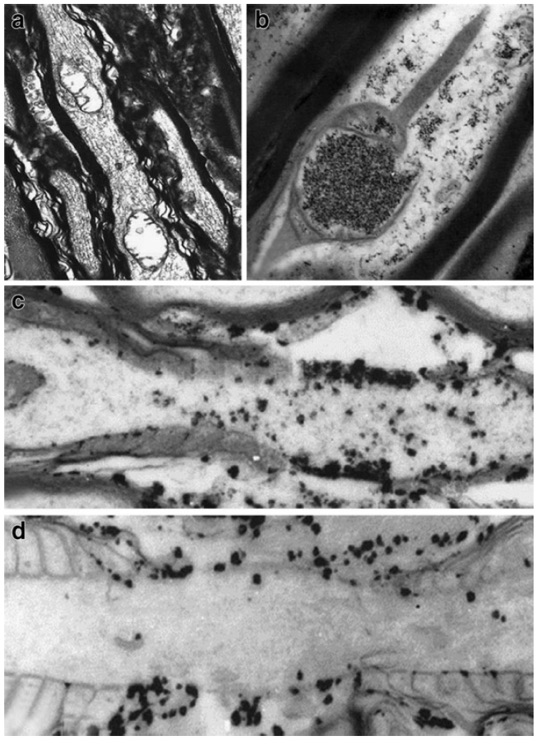
Figure 2 demonstrates evidence of calcium petrification of mitochondria in the upper right image with subsequent vacuolization in the upper left image in an animal model of mTBI. In addition, the lower two images represent pre- and post-disruption of the sodium/potassium pump following axonal stretching in the same model. Chronically, this ongoing disease process may progress to involve neurodegenerative changes associated with amyloid and tau protein, as well as parenchymal loss (atrophy). (6,7)
On the macro level, Bigler (2013) demonstrated these neurodegenerative changes over time via sequential MRI imaging in a patient with moderate TBI. (6) (Figure 3). Other studies indicate an increased risk of depression over time. (8,9)
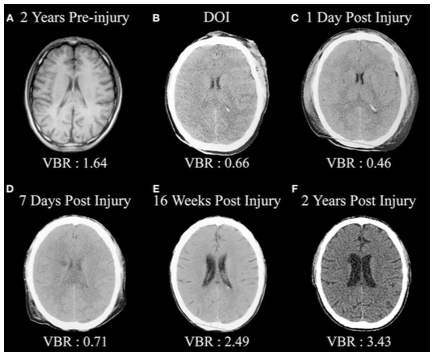
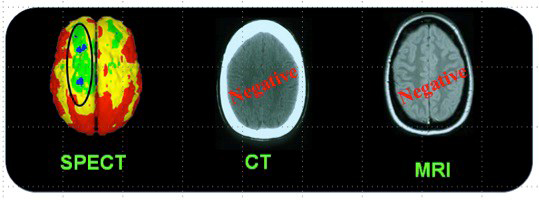
Figure 4 presents the case of a woman who sustained a mTBI in a rear-end car accident (without loss of consciousness). At the ED, CT imaging of head was normal and she was sent home. Unfortunately, she developed PPCS and sought neurologic opinion six months following the accident. An MRI of her brain was normal. Her PPCS symptoms persisted two more years. Brain SPECT imaging then revealed significant metabolic rCBF abnormalities underlying her PPCS. Studies have consistently demonstrated the superior sensitivity of functional imaging, such as SPECT and/or PET over CT and/or MRI to evaluate mTBI in the chronic setting. (10,11)
Evaluation of TBI
A careful history of any alternation in state of consciousness following a traumatic event becomes critically important in the acute setting. The presence of life-threatening orthopaedic and internal injuries often obscures this process. Neuro assessments like “grossly intact” and “Ox4” are not adequate. Skilled clinicians explore the possibility the patient was “dazed” or “disoriented” and document such. A normal GLASCOW score (13 to 15) does not rule out the presence of a mTBI nor does a “normal” head CT.
Treatment of TBI
Initial treatment for mTBI in athletics is removal from the activity and implementation of standardised concussion protocols. Discharge from the ED with a prescription to “rest” is standard. For patient developing PPCS, standard treatments include pharmacotherapy, cognitive rehabilitation, speech, physical, vision and vestibular therapies. Unfortunately, they are minimally successful. (12)
Recently, modalities including neurofeedback, transcranial magnetic stimulation (TMS), hyperbaric oxygen therapy (HBOT) and photobiomodulation therapy (PBMT) have shown promise. However, only HBOT and PBMT have demonstrated the ability to actually upregulate and downregulate neuron genetic mechanisms. (13-15) These modalities appear to be equally effective in reversing the effects of mTBI on brain SPECT imaging. (13,15)

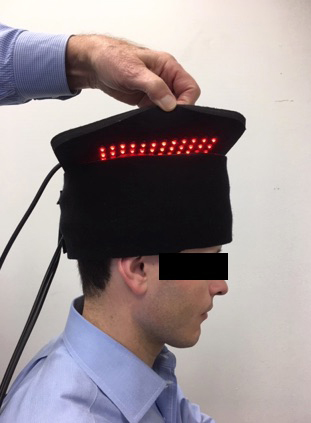
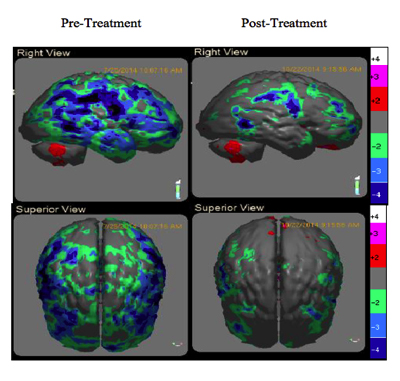
Uniquely, the proposed mechanism of PBM is the release of nitric oxide from cytochrome C-oxidase (COX) which increases ATP, genetic signalling to upregulate anti-inflammatory, local circulation, cell growth and protective factors. (7) (Fig 5) These processes promote improved brain function and subsequent clinical improvement. In a case series of 12 veterans with chronic mTBI lasting greater than 18 months, Hipskind et al demonstrated statistical improvements in cognitive function and rCBF using quantitative SPECT following PBMT. (Fig 7) This study used an FDA cleared InLight Medical PBM device (Fig 6) which pulsed both red (629nm) and near-infrared (850 nm) light transcranially for 20 minutes thrice per week for six weeks. Standard neuropsychological testing revealed improvements in 14 out of 15 subscales, with six of the 14 reaching statistical significance. In addition, quantitative brain SPECT imaging revealed improvements in rCBF in nine of 12 subjects. Subgroup statistical analysis of the nine with improved rCBF revealed quantitative blood flow improvements at the P<0.007 level. Although not formally evaluated, all subjects reported subjective improvements in mood, attention and sleep. There were no reported adverse effects.
In conclusion, chronic mTBI is a very common and underdiagnosed source of morbidity and mortality in the US. Standard anatomical imaging with CT and MRI modalities are less sensitive to the neuropathological changes of mTBI in the chronic setting than functional brain imaging such as SPECT. Given its ability to penetrate the skull and address the neuronal mitochondrial dysfunction seen in mTBI, PBMT offers a non-toxic, non-invasive, safe and effective alternative to standard treatments. With its low cost and the convenience of in-home use, PBMT seems ideally positioned to address the silent epidemic of mTBI in the US.(14, 15)
References
- Faul M, X.L., Wald M. M, Coronado V. G. , Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002–2006. . Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, 2010.
- Kenzie, E.S., et al., Concussion As a Multi-Scale Complex System: An Interdisciplinary Synthesis of Current Knowledge. Front Neurol, 2017. 8: p. 513.
- McAllister, T., Neuropsychiatiric sequelae of head injuries. The Psychiatric Clinics of North America, 1992. 15(2): p. 395-423.
- Christopher A. Taylor, M.J.B. Jeneita M. Bell, and Likang Xu, Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths – United States, 2007 and 2013. MMWR, 2017. 66.
- Giza, C. and D. Hovda, The new neurometabolic cascade of concussion. Neurosurgery, 2014. 75 Suppl 4: p. S24-33.
- Bigler, E.D., Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci, 2013. 7: p. 395.
- Chung, H., et al., The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng, 2012. 40(2): p. 516-33.
- Jesse R. Fann, M., MPH; Bart Burington, MS; Alexandra Leonetti, MS; Kenneth Jaffe, MD; Wayne J. Katon, MD; Robert S. Thompson, MD, Psychiatric Illness Following Traumatic Brain Injury in an Adult Health Maintenance Organization Population. Arch Gen Psychiatry, 2004. 61: p. 53-61.
- Ricardo E. Jorge, M.R.G.R., MD; David Moser, PhD; Amane Tateno, MD; Benedicto Crespo-Facorro, MD; Stephan Arndt, PhD, Major Depression Following Traumatic Brain Injury. Arch Gen Psychiatry, 2004. 61: p. 42-50.
- Davalos, D.B. and T.L. Bennett, A review of the use of single-photon emission computerized tomography as a diagnostic tool in mild traumatic brain injury. Appl Neuropsychol, 2002. 9(2): p. 92-105.
- Raji, C.A., et al., Clinical utility of SPECT neuroimaging in the diagnosis and treatment of traumatic brain injury: a systematic review. PLoS One, 2014. 9(3): p. e91088.
- Henderson, T. and L. Morries, SPECT Perfusion Imaging Demonstrates Improvement of Traumatic Brain Injury With Transcranial Near-infrared Laser Phototherapy. Advances in Mind-Body Medicine, 2015. 29(4): p. 27-33.
- Hipskind, S.G., et al., Pulsed Transcranial Red/Near-Infrared Light Therapy Using Light-Emitting Diodes Improves Cerebral Blood Flow and Cognitive Function in Veterans with Chronic Traumatic Brain Injury: A Case Series. Photomed Laser Surg, 2019. 37(2): p. 77-84.
- Hamblin, M.R., Shining light on the head: Photobiomodulation for brain disorders. BBA Clin, 2016. 6: p. 113-124.
- Harch, P.G., et al., Case control study: hyperbaric oxygen treatment of mild traumatic brain injury persistent post-concussion syndrome and post-traumatic stress disorder. Med Gas Res, 2017. 7(3): p. 156-174.
Please note: This is a commercial profile