Global clinical diagnostics company Novacyt introduce a new automated liquid handling system to meet growing demand for no manual pipetting in COVID-19 PCR testing
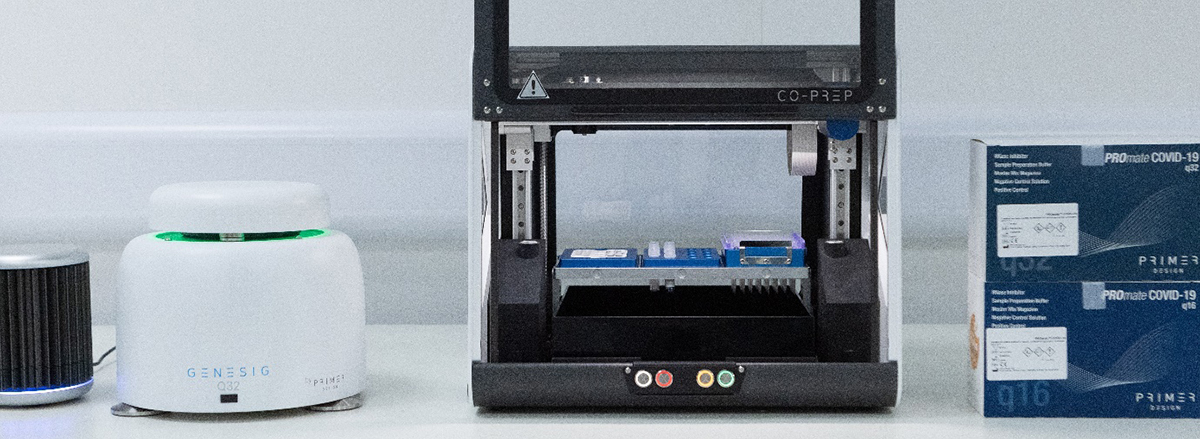
The CO-Prep™ Automated Liquid Handling System is for use with the PROmate® COVID-19 assay workflows, designed to support busy laboratories and private healthcare providers to perform efficient COVID-19 testing.
CO-Prep™ optimises liquid handling for PROmate®COVID-19 assays, an established total workflow solution, which includes sample preparation, qPCR amplification, and analysis on the genesig® q16 and q32 qPCR instruments. This is specifically for the qualitative detection of nucleic acid from SARS-CoV-2 from anterior nasal and throat samples prepared on dry swabs.
These direct-to-PCR assays require only one pipetting step – manual until now – to transfer 5 µl of sample into the 0.1 ml PCR tube, ready for amplification. With CO-Prep™, this one pipetting step is automated, which reduces the risk of contamination and improves the accuracy of result by removing human experimental variation.
Rebecca Underwood, Novacyt’s Senior Product Manager for the new CO-Prep™ system, said: “We were asked to address the need for no manual handling of the pipetting process, and with CO-Prep™, by automating the pipetting, we’re creating walkaway time, freeing up the operator to do other tasks.”
Sample tracking
A key feature of the system is its ability to track each patient sample throughout the workflow, from swab to results. Sample/patient barcodes, as well as the QR codes on the setup plates, can be scanned to upload the information to the CO-Prep™ software. QR barcodes are provided in PROmate® kits. Once the samples have been prepared for qPCR amplification, the sample/patient IDs are transferred easily across to the genesig® software, ready for the results to be recorded. The CO-Prep™ software application also has an option to create sample queues, so that multiple setup plates can be prepared in advance. This helps to increase sample throughputs and delivers additional walkaway time.
Mark Coldwell, Novacyt’s Head of Scientific Communications, states: “The whole CO-Prep™ system has been designed to be as user-friendly as possible, providing accurate and precise automatic pipetting. Coming from an academic testing environment myself, I can understand the need to make the laboratory workflow more efficient, and CO-Prep™ requires minimal training – which we provide on setup – before it becomes an integral part of everyday use. The software is simple and intuitive to use and gives busy laboratories the means to increase daily throughput.”
The CO-Prep™ software application has been designed for ease-of-use, to allow runs to be quickly set up, queued, and executed with minimal steps within a simplified workflow. It tracks and records samples, setup plates and unique magazine identifiers to ensure traceability of clinical samples. The system also shows run progress and provides alerts when sample preparation is complete. CO-Prep™ requires a PC/laptop/tablet to operate it, but it does not require a dedicated I.T. system; the same PC/ laptop can run both the CO-Prep™ and the genesig® software simultaneously.
Mobility
Another CO-Prep™ attribute is its compact size. It was designed with a small footprint that minimises laboratory bench space or fits easily into a Class II Biological Safety Cabinet (BSC) to suit individual requirements, particularly if the testing is being carried out in the field or near-to-patient.
Easy cleaning
The system has an inbuilt UV LED light function for fast, chemical free decontamination following cleaning. UV exposure time can be customised to meet individual requirements and a record is saved in the CO-Prep™ software. The system has two tip waste containers which can easily be removed and cleaned.
“CO-Prep has ease-of-use and expandability,” says Mark. “It’s ideal for those who want to start small but then scale; it’s very portable.”
The PROmate® evolution
PROmate® itself has also evolved to meet changing customer demand. Launched 18 months ago, it was designed to further improve the workflow efficiency of COVID-19 testing when used in combination with Novacyt’s q16 and q32 instruments. The reagents involved in COVID-19 RNA extraction and PCR test products were repackaged, to reduce the number of consumables and steps required. This cut operator complexity and improved cycle times. PROmate® uses a viral inactivation methodology validated by Public Health England for potential use outside of laboratory environments.
CE Mark approved, the original one-gene target test has now been supplemented by a two-gene version, in response to evolving need during the COVID-19 pandemic. More recently, the PROmate® COVID-19 2G, that can detect two SARS-CoV-2 targets within ORF1ab, has been validated by the UK Health Security Agency and given Coronavirus Test device Approval (CTDA) on its register of approved devices for COVID-19.
Novacyt maintains one of the world’s leading bio-surveillance systems, with daily monitoring to identify emerging variants of concern. Weekly reports are produced, to ensure that all Novacyt COVID-19 assays, including the PROmate® workflow, continue to perform excellently.
For a free demonstration on the PROmate® and CO-Prep™ workflows, kindly contact enquiries@primerdesign.co.uk.
Please note: This is a commercial profile
© 2019. This work is licensed under CC-BY-NC-ND.