Dr Anne Hansen, Associate Professor of Pediatrics at Harvard Medical School, exposes neonatal hypothermia as a silent killer and discusses what can be done to prevent the condition
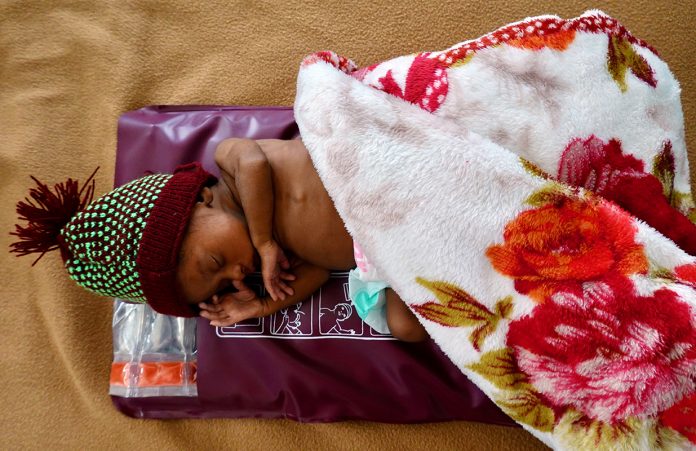
Neonatal hypothermia is widely recognised to be one of the major causes of preventable morbidity and mortality, especially for the world’s poorest newborns. It is estimated to contribute to 40% of the 2.7 million babies who die each year, almost exclusively in low- and middle-income countries (LMIC). There is a direct correlation between the degree of hypothermia and its associated mortality risk. One study found an 80% increase in mortality rate for every 1oC decrease in the first measured infant temperature. Another estimated that premature infants with a core temperature under 35oC have at least a tenfold increased risk of mortality. Survivors of hypothermia are at risk for multiple morbidities, most concerningly stunted growth, including brain growth, increasing the risk of neurodevelopmental impairment in this already vulnerable population. Thus, the thermal environment is one of the few modifiable risk factors for at risk newborns.
The incidence of hypothermia is highest in premature/Low Birth Weight (LBW) newborns, of whom 20 million are born each year, over 96% in LMICs. Small and sick newborns tend to cool towards the temperature of their environment, making it challenging to keep their temperature in a normal range even in hot ambient conditions such as sub-Saharan Africa. This large at-risk population is dependent on an external heat source for the weeks to months it may take until the infant develops the fat and metabolism needed to independently maintain euthermia.
The heat chain
In rich countries, this heat is provided by incubators and warming tables. These are expensive, difficult to use, clean and maintain, and require a constant source of electricity. The WHO recommends Skin to Skin (STS) care, placing the baby directly on the chest, typically of the mother. This is a low cost, highly effective intervention that must be supported. Yet, a supplemental external heat source is needed when STS does not provide sufficient heat, or when the mother is unavailable. Additionally, health care providers may need access to an ill baby for assessment and treatments. Thus, a technology to complement STS is urgently needed.
UNICEF and PATH have described the characteristics required of an optimal thermoregulatory device for the low resource setting. Both insist that the device:
- Generates an accurate temperature.
- Has a safety feature to avoid use when too hot.
- Has a biocompatible, non-toxic patient interface.
- Is easy to use and clean.
- Is durable and re-usable with minimal consumables.
- Is appropriate for patient transfer.
- Allows the patient to be visible and accessible.
Commercially available options do not meet these standards, each having significant drawbacks. Specifically, in the area of conduction mattresses, the products are either relatively expensive or difficult to prepare or clean due to attached fabrics in an environment with few diapers or clothes washers.
An innovative complement to STS
In response to this unmet urgent need, Dr Hansen and her colleagues developed a Durable Re-usable Electricity-free Affordable Mattress called the DREAM Warmer. It is a heating pad made of phase change material that is melted by boiled water and then stays at skin temperature for approximately six hours. It can be used on its own, or in addition to STS for maximal heat provision. Both options keep the mother and baby together. It satisfies all the major characteristics of both UNICEF and PATH. It is intuitive to prepare, use, and clean, requiring minimal training. Simple instructions are provided on the insulating pad and thermos to ensure comprehension, even by those with low literacy skills. It is intended for use in hospitals, health centres, on transport, or in the home setting.
Field-tested
To assess the safety, effectiveness and feasibility of the Dream warmer, two pilot studies (1,2) followed by a multi-centred prospective cluster randomised stepped-wedge study (3) were conducted in rural hospitals across Rwanda. Combining all three studies, the Dream Warmer has now undergone over 1,000 uses and demonstrated excellent safety, feasibility and effectiveness. There were no adverse side effects such as burns, rashes or other skin irritation. There were no instances in which nurses prepared, used or cleaned it incorrectly. The warmer left only 11% of babies hypothermic, and those who used the warmer had a mortality rate one-third that of those who never used the warmer.
Ready for scale
There is now very strong evidence for using the Dream Warmer on a wider scale and Dr Hansen and her team are confident their product will be an invaluable addition to the treatment of hypothermia in LMICs. With the appropriate equipment, neonatal hypothermia is a preventable condition and should be a key focus to reduce neonatal mortality and ensure that these vulnerable patients not only survive, but thrive.
References
- Nahimana E, May L, Gadgil A, Rapp V, Magge H, Kubwimana M, Nshimyiryo A, Kateera F, Feldman H, Nkikabahizi F, Sayinzoga F, Hansen AR. A low cost, re-usable, electricity-free infant warmer: Evaluation of safety, effectiveness, and feasibility. Public Health Action. 2018 Dec; 8(4): 211-217.
- May L, Nshimyiryo A, Kubwimana M, Nahimana E, Schoen N, Gadgil A, Kateera F, Feldman H, Nyishime M, Hansen AR. Performance of a non-electric infant warmer in Rwandan health centers. Global Pediatric Health. 2019 Oct; 6: 1-10.
- Uwamariya J, Mazimpaka C, May L, Nshimyiryo A, Feldman H, Sayinzoga F, Umutesi S, Gadgil A, Rapp V, Nahimana E, Hansen AR. Safety and effectiveness of a non-electric Infant Warmer for hypothermia in Rwanda: A cluster-randomized stepped-wedge trial. Lancet: EClinical Medicine. 2021 Apr; 34(100842). PubMed PMID: 33997734.
Please note: this is a commercial profile
© 2019. This work is licensed under CC-BY-NC-ND.