Here, Prof Kazunari Domen, Shinshu University & The University of Tokyo, explains a large-scale renewable hydrogen production system based on arrayed photocatalytic water splitting panels & an oxyhydrogen gas-separation module under development for practical solar hydrogen production
Water splitting reaction driven by sunlight is a technology for producing renewable solar hydrogen on a large scale. Particulate photocatalyst-based systems are expected to be easily scaled up because they can be spread over a large area by using inexpensive methods (1). In fact, the use of a panel reactor allows a particulate photocatalyst to split water into hydrogen and oxygen under natural sunlight without deteriorating the performance, even if it is expanded to the one-square-metre scale (2).
One of the next challenges is to demonstrate the scale-up of the panel reactor, the separation and recovery of solar hydrogen from the product moist oxyhydrogen gas and the long-term stability of the system in the outdoor environment. Prof Domen’s group has designed extensible photocatalyst panel reactors and devised gas-separation modules that can safely separate hydrogen and oxygen (3).
Large-scale photocatalyst panel reactor
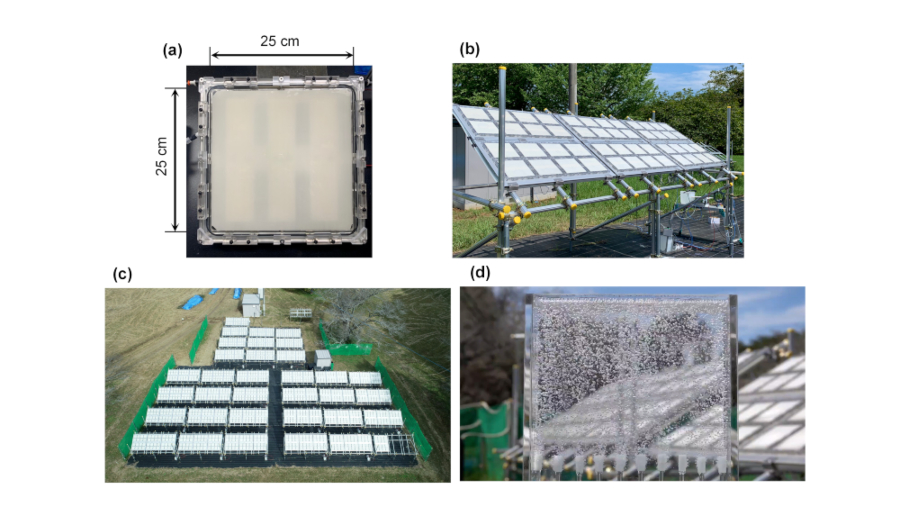
The newly-designed photocatalyst panel reactor has a transparent glass window and contains a 25 cm square strontium titanate (SrTiO3) photocatalyst sheet (Fig. 1a). The gap between the window and the photocatalyst sheet is 0.1 mm and is filled with water. The SrTiO3 photocatalyst can use only the ultraviolet light of sunlight, but it can decompose water into hydrogen and oxygen efficiently, with a quantum yield of almost unity (4). Therefore, photocatalyst sheets for water splitting can be readily obtained, for example, by spraying slurry of the photocatalyst on substrates.
Adapted from Nishiyama, H., Yamada, T., Nakabayashi, M. et al. Photocatalytic solar hydrogen production from water on a 100 m2-scale. Nature (2021). https://doi.org/10.1038/s41586-021-03907-3.
Copyright © 2021, The Authors, under exclusive licence to Springer Nature Limited.
The panel reactors are assembled into 3 m2 scale modules (Fig. 1b), and the modules are further connected by small diameter plastic tubes to assemble a 100 m2 scale photocatalyst panel reactor (Fig. 1c). The hydrogen-oxygen mixed gas generated from the 100 m2 scale panel reactor can be observed as bubbles bursting out vigorously when introduced into a water tab (Fig. 1d). The large photocatalyst panel can decompose water under natural sunlight with a solar-to-hydrogen energy conversion efficiency of 0.76% in the summer when sunshine conditions are good.
Gas-separation module
The product gas from the photocatalyst panel reactor contains hydrogen and oxygen in a ratio of 2:1 in addition to water vapour. Therefore, it is necessary to separate hydrogen. Here, the product gas is processed by a gas-separation module incorporating a commercial polyimide hollow fibre separation membrane. Hydrogen is concentrated in the filtrate gas, and oxygen is concentrated in the residual gas.
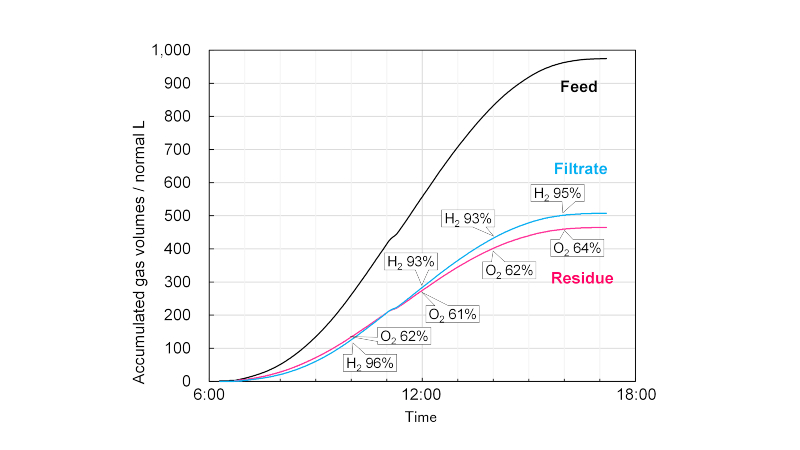
The gas-separation module is equipped with tanks that temporarily store the product gas and valves that autonomously adjust the gas feed rate to the separation membrane constant to stabilise the separation performance. An example of one-day operation of the gas-separation module connected with the 100 m2 scale photo-catalyst panel reactor is presented in Fig. 2. The daily production of humid oxyhydrogen gas was 970 L. From this, 505 L of filtrate gas with a hydrogen purity of 94% was separated, and the hydrogen recovery rate was 73%. The separation performance has been shown to be maintained for a long time, regardless of the weather and season.
Safety issues
Hydrogen mixed with oxygen is prone to explosion. At present, not only the oxyhydrogen gas generated by the photocatalyst panel reactor, but the filtrate and residue gases processed by the gas separator are still within the explosive range. Therefore, it is necessary to pay close attention to safety and reduce the risk of explosions. Fortunately, the photocatalyst panel reactor, gas collection piping, and hollow fibre separation membrane are not damaged or deteriorated in performance even when the oxyhydrogen gas contained inside is intentionally ignited.
The tanks (3 L in volume) for the temporary storage of the product oxyhydrogen gas can withstand an explosion and be reused by providing an appropriate partition inside. The results suggest that even highly explosive oxyhydrogen gas can be safely handled using a well-designed system although more rigorous testing is certainly needed.
Future challenges
The demonstration of the large-scale photocatalytic water splitting and gas collection and separation indicates that scaling of solar hydrogen production via photocatalytic overall water splitting to a size of 100 m2 is feasible, with further scaling in principle possible without efficiency degradation.
At the same time, this demonstration highlights future challenges associated with the installation of this technology, including the development of photocatalysts efficiently splitting water under visible light and membranes more efficiently separating hydrogen, reduction of system construction cost, improvement of energy efficiency of water and gas processing, and ensuring the safety of the entire system and process in compliance with laws and regulations. The cooperation of experts in various fields will be required for establishing cost-effective large-scale renewable solar hydrogen production systems.
References
(1) Hisatomi et al., Nature Catal. 2, 387 (2019).
https://www.nature.com/articles/s41929-019-0242-6
(2) Goto et al., Joule 2, 509–520 (2018)
https://www.sciencedirect.com/science/article/pii/S2542435117302246
(3) Nishiyama et al., Nature, in press (2021)
https://doi.org/10.1038/s41586-021-03907-3
(4) Takata et al., Nature 581, 411–414 (2020)
https://www.nature.com/articles/s41586-020-2278-9
Please note: This is a commercial profile
© 2019. This work is licensed under a CC BY 4.0 license.