Research suggests that multisensory integration could be used as a novel marker for preclinical Alzheimer’s disease given reported associations between magnitude of visual-somatosensory integration and important cognitive and motor outcomes
“If we can detect modifiable multisensory impairments, then we can develop novel strategies to strengthen these processes, in an effort to slow the progression of Alzheimer’s disease and prevent serious mobility problems such as falls.”
Jeannette R. Mahoney, PhD
Alzheimer’s disease
Alzheimer’s disease affects nearly 6 million Americans and is the most common cause of dementia.[1] The notion that Alzheimer’s alters sensory processing is in its early stages.[2] This premise is supported by growing evidence that amyloid-beta (Aβ) protein accumulates in sensory-association areas of the brain well before higher-order cognitive areas like the prefrontal cortex (PFC).[3]
The National Institute on Aging (NIA) has recognised that functional changes in sensory and motor systems modulate the progression of Alzheimer’s disease, and have requested discovery of novel, non-cognitive and non-invasive predictors. Our research reveals that worse visual-somatosensory integration is associated with worse motor – specifically worse balance, slower gait, and increased falls – and worse cognitive functioning. Thus, further investigation of whether multisensory integration could be developed not only as a novel behavioural marker of Alzheimer’s disease, but also as a novel marker of falls, is warranted.
Multisensory Integration (MSI)
When sensory inputs (e.g., visual and somatosensory) are presented concurrently, they combine in the brain by a non-linear process to yield faster responses than their unisensory constituents. Researchers claim that our brains are designed to simultaneously process multiple sensory inputs so as to produce the most appropriate response to environmental cues.[4] Research on animals (cats and primates) and humans (young adults) reveals that intact multisensory integration is critical for functional independence in the real world, including successful completion of daily activities.[4-6]
Further, studies reveal that flexible multisensory integration is regulated by PFC, including dorsomedial and ventrolateral subregions.[7, 8]
Much of the research conducted over the past several decades has investigated multisensory integration effects (namely auditory-visual, auditory-somatosensory and visual-somatosensory combinations) in young adults, using diverse psychophysical, electrophysiological, and neuroimaging techniques.[4] Findings typically reveal a speeding up in response time [also known as reaction time (RT) facilitation] for the multisensory as compared to the unisensory events. Considerable strides have been made in defining the underlying properties, neural substrates, and timing of multisensory integration effects in healthy young adults.[4] However, influential editorials highlight limited multisensory aging research, as well as the lack of clinical multisensory applications as major gaps in the field.[5, 6, 24]
MSI & Aging
Further understanding of multisensory integration in aging could prove valuable, given that increased sensory deficits are associated with aging. Age-related visual and somatosensory impairments have been linked to slower gait,[9] functional decline,[10] increased risks of falls,[11-14] and poorer quality of life.[15] In a study contrasting multisensory integration in old and young adults across auditory-visual, auditory-somatosensory, and visual-somatosensory combinations, we reported that older adults exhibit the greatest (i.e., best) multisensory integration effects when presented with visual-somatosensory stimulation.[16] We generated evidence for robust, but differential visual-somatosensory integration (VSI) effects in healthy older adults [17-19] and discovered its significant link with clinically-meaningful outcomes like falls, balance, gait speed, and cognition.[17-22]
Specifically, we found that older adults with intact levels of VSI (area-under-the-curve values > 0 over fastest 10 percent of RTs) demonstrate better balance, faster gait velocity and lower incidence of falls, compared to those with VSI deficits (area-under-the-curve values < 0 over fastest 10 percent of RTs). VSI deficits associated with worse unipedal stance and worse spatial gait performance inevitably contribute to worse quality of life and increased risk for falls. Additionally, we reported that older adults with mild cognitive impairments and dementia demonstrated significantly reduced magnitude of VSI compared to older adults without cognitive impairments.
Clear clinical-translational value
Relatively few aging studies to date have investigated the clinical utility of multisensory tools. Our method for quantifying multisensory integrative effects demonstrates clear clinical-translational value with regards to predicting declines in mobility, including but not limited to falls. Using our research as a solid foundation and our patent-pending system and methods for testing multisensory integration effects (U.S. Provisional Application No: 62/908,180; U.S. Non-Provisional Application No: 17/038,974), we have developed an innovative and quantitative visual-somatosensory iPhone-based reaction time assessment called CatchUTM [see 23].
The main objectives of the CatchUTM simple reaction time test and corresponding personalised physician report is to facilitate the identification of patients who are at increased risk of falls and promote physician-initiated falls counselling or other therapeutic services. We believe that optimising integration of visual-somatosensory inputs may ultimately provide the necessary framework for development of future personalised multisensory interventions aimed at reducing falls and improving mobility, while alleviating disability, that will in turn help our seniors maintain functional independence.
Recent Funded Projects
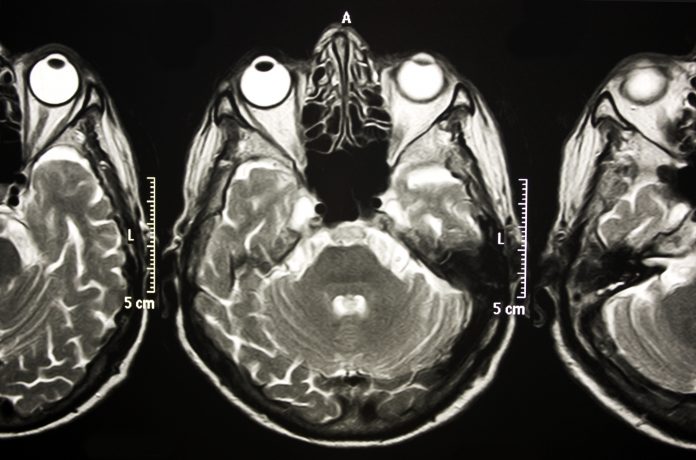
The functional neural substrates of VSI have not been identified in either healthy or cognitively impaired adults. In our most recent project funded by the NIA (1R01AG075679),we aim to establish VSI as a novel marker of preclinical Alzheimer’s disease since growing evidence suggests that Alzheimer’s pathology manifests in sensory association areas well before appearing in neural regions involved in memory function. Specifically, we plan to examine integrative neural activation using state-of-the-art multimodal MRI & fMRI procedures in older adults with and without preclinical Alzheimer’s disease, while linking these specific neural networks to clinically meaningful outcomes like falls. This project specifically challenges existing multisensory and aging research paradigms by assessing the time course and interplay between cognitive, motor, and sensory processing in healthy and pathological aging. Identifying associated functional neural networks involved in multisensory integration processes will allow us to conduct future randomized
clinical trials to determine whether novel multisensory-based interventions can alleviate disability in people with cognitive impairments.
Disclosures:
JRM has a financial interest in JET Worldwide Enterprises Inc., a digital health startup spun out of research conducted at Albert Einstein College of Medicine.
*Please note: This is a commercial profile
© 2019. This work is licensed under CC-BY-NC-ND.