Here we learn from Biljana Stangeland, PhD, Executive Director, Chief Scientific Officer and Co-founder at Alv B AS, about exciting new developments in therapeutic feline and canine cancer vaccine
Canine cancer bear substantial similarities with their human counterparts[1-4]. Treatment of pets that get solid tumours will typically consist of surgery, chemotherapy and sporadically radiation, or a combination of these. Unfortunately, these treatments are not always effective.
In humans, experimental cancer treatments have had a slow but sure advance into the clinics and have given many patients new hope. Especially, treatment methods related to immunotherapy have been on the rise for the past decade.
Despite the documented clinical success of human immunotherapy, the opportunities to apply these treatments on companion dogs in clinical settings are few or non-existent in Nordic countries. As the number of insured pets in these countries increases so does the prospect that experimental treatments will also be affordable for pet owners.
Recent research on pet cancers shows that cancer immunotherapy can have life-prolonging effects and is often associated with fewer side effects (for the overview of literature see the provided reviews and the references therein)[4,5]. We are therefore developing new therapies for companion dogs (and cats) that will combine our expertise in human immunotherapy and translational research with the newfound knowledge of canine cancer at the molecular level.
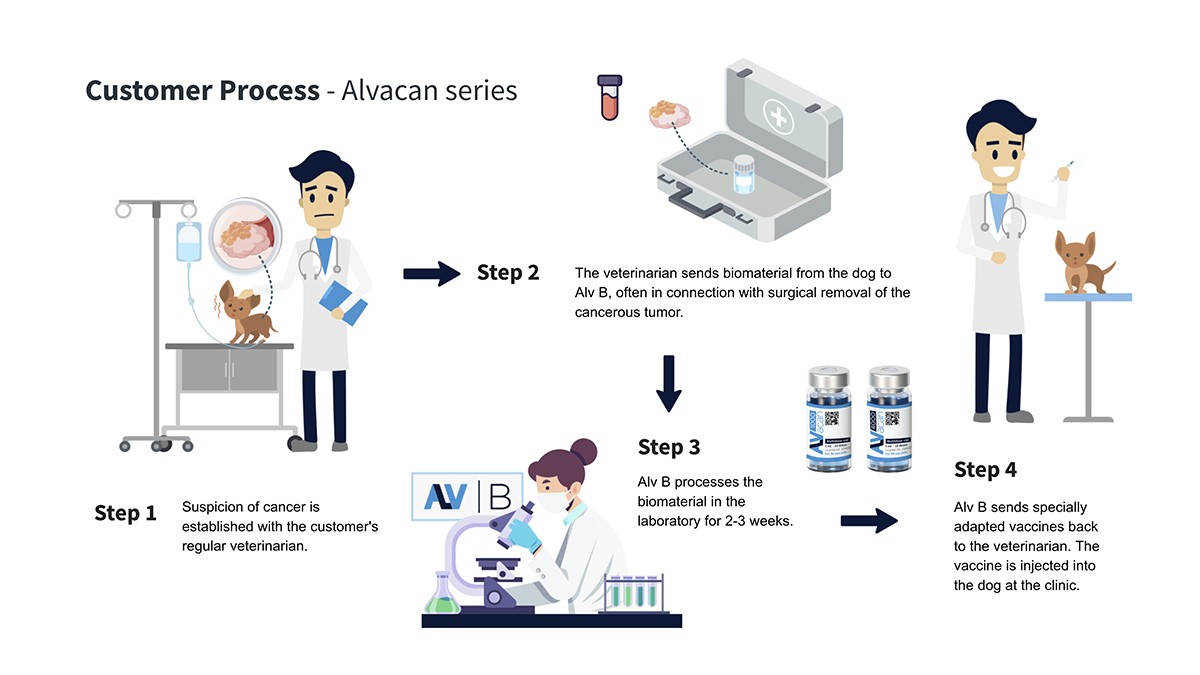
Alvacan (Figure) is our product line of therapeutic cancer vaccines that combines the best of classical cellular immunotherapy with recombinant DNA/RNA technology.
The treatments that we are developing are tailored for each individual patient and will serve as a supplement to standard cancer surgery and chemotherapy. We are currently testing two immunotherapy treatments that can be administered at the local veterinary clinic.
What we need is a patient’s blood and if possible, a cancer tissue sample that is typically obtained through surgery (Figure). The administration of vaccines and patient follow up will continue in the selected partner clinics. These vaccines will be affordable for the typical pet owner in Nordic countries.
Why is biobanking so important for feline & canine cancer vaccine research?
As a part of our vaccine research, we have created a biobank of living tissues and cell lines from canine and feline solid tumours. This collection serves as a base for our research and can be available for other researchers through collaboration with us or commercialisation of our material. We have already successfully adapted our previously described «human» methods to work with canine and feline biobanking and cell cultures [6-10].
For each primary cancer cell line, isolated from patient biopsies, we will attempt to establish a cell line that is enriched for cancer stem cells (CSCs). CSCs are believed to be cellular drivers of carcinogenesis. One of the hallmarks of CSCs is self-renewal. We have previously extensively studied stemness and growth properties in a series of primary CSC lines [10]. Our biobank comprises currently 180 samples of 80 canine and feline patients and these numbers are growing day by day.
By the end of this year, we expect to have doubled the number of patient samples. Our collection of living tissues and cell lines includes intracranial, mammary, and testicular solid tumours, sarcomas and many other less prevalent cancers.
We will also map molecular profiles of canine tumours using the previously established techniques tested in human cancer [10]. Once such information is available, we can develop more advanced targeted treatments. By comparing molecular profiles from many cancer patients, we can form a more general picture of the disease and develop treatments with a broader effect.
The need for animal models that translate to human immunity is a primary challenge of human cancer immunotherapy[11]. A better understanding of canine carcinogenesis is therefore one of the main goals of our research. This project has excellent translational potential and will not only be beneficial for cancer-suffering pets, but also humans.
We are also studying gene expression regulation in canine cancer. Targeted proteomics and gene expression analysis is conducted according to methods that we previously established in humans[10]. There are currently only a few bioinformatic tools that apply to canine genomes, transcriptomes and proteomes. The number of public canine molecular profiles is also far behind that for human and murine counterparts.
Our goal is to generate molecular data profiles and design bioinformatic tools that will speed up this type of research and contribute to the information exchange between research organisations and communities. We intend to generate many canine molecular cancer profiles and create new tools for data analysis. Based on a better understanding of molecular drivers of canine carcinogenesis we are also planning on building next-generation diagnostic tools for canine cancer. We are going to combine molecular profiles with clinical and imaging data to design machine learning and AI tools that will enable better diagnostics and improve medication efficiency.
Literature list
- Sammarco et al., (2020) doi:10.1177/0300985820948823
- Gustafson et al. (2018) doi:10.1016/j.pharmthera.2018.01.012
- Sumner et al. (2021) doi:10.1038/s41598-021-86805-y
- Tarone et al. (2019) doi:10.1007/s00262-019-02360-6
- Dow S (2019) doi:10.3389/fimmu.2019.02935
- Behnan J, Stangeland B et al., (2017) doi:10.1038/onc.2016.230
- Behnan J, Stangeland B et al., (2016) doi:10.1038/srep32788
- Behnan J, Stangeland B et al., (2017) doi:10.1038/cddis.2017.368
- Palmero et al., (2016) doi:10.1038/srep39394
- Stangeland et al., (2015) doi:10.18632/oncotarget.4613
- Hegde PS, Chen DS (2020) doi:10.1016/j.immuni.2019.12.011
About the author
Biljana Stangeland. PhD is an Executive Director, Chief Scientific Officer, and co-founder of Alv B AS (https://alvb.no/), a Norwegian biotech start-up. Biljana has more than 25 years of academic research behind her and over five years as a Lead Data Scientist in the private sector.
Please note: This is a commercial profile
© 2019. This work is licensed under CC-BY-NC-ND.