S. Caserta1*, S. Guido1, M. Mugnano2, G. Condorelli3, V. de Franciscis4, V. Cristini5
1 Dipartimento di Ingegneria Chimica dei Materiali e della Produzione Industriale, Federico II Napoli Italy. CEINGE-Advanced Biotechnology
2 Institute of Applied Science & Intelligent Systems (ISASI) “E. Caianiello” – National Research Council, Pozzuoli (NA), Italy
3 Department of Molecular Medicine and Medical Biotechnology, Federico II University of Naples, Italy
4 IEOS-CNR Italy
5 Houston Methodist, US
* sergio.caserta@unina.it
The use of a trans-disciplinary approach to cancer research is necessary for complex systems like tumors
Tumors are extremely complex systems, involving biological interactions of pathological and normal cells with the extracellular biochemical microenvironment, where all important transport processes, such as nutrients delivery and waste removal, play a key role as well. Recapitulating all these factors into a comprehensive modelling framework is a formidable task in cancer medicine.
Different research approaches are typically proposed, including in-vivo, in-vitro, in-silico, and mathematical models to study cancer growth and treatment. Each of these methodologies alone can only provide and examine a partial description of the interplay between complex microenvironment and drug transport efficiency, and hardly allows to provide patient-specific information, to be used to design personalized precision treatments.
We believe a trans-disciplinary experimental-theoretical approach needs to be implemented to identify quantitative mechanistic correlations between tumor stromal/microenvironmental parameters and cell behavior. The results will provide insight on resistance factors that limit drug delivery for cancer therapies, and suggest the design of novel nano-carriers to enhance drug transport and release by integrating in vitro, theoretical, and in vivo models, superior to any actually existing experimental approach.
We are developing a novel Elementary Tissue Unit (ETU) based on spatial and temporal microfluidic technology integrated with ex vivo 3D co-cultures and advanced mathematical modelling, enabling us to deliver the full potential of each technology and their combination toward predictive precision medicine. We have set up 3D cultures of patient-derived breast cancer organoids. The latter are miniature forms of tissues that exhibit three-dimensional architecture and are able to maintain phenotypical properties when cultured in a dish.
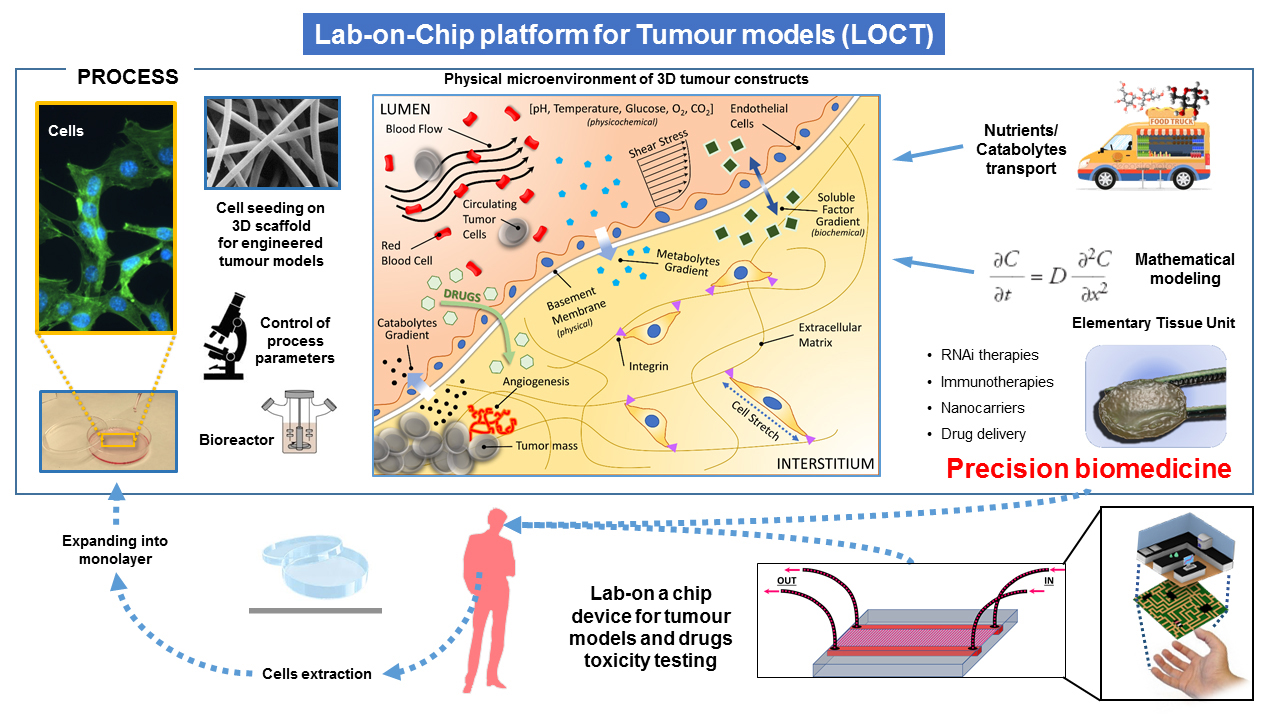
The proposed integrated experimental-theoretical approach overcomes the limitations of typical in-vivo, in-vitro, in-silico models, when taken alone, since it is based on a highly interdisciplinary collaborative effort of engineers, mathematicians, biologists, chemists and oncologists exceeding research activities that can be offered by each expertise individually.
Advanced mathematical models can be used to abstract key parameters responsible of the description of cancer growth, evolution and treatment. The same parameters can be reproduced in an ex-vivo model, where advanced microfabrication techniques can be used to reproduce the microenvironment surrounding elementary tissues in the patient body mimicking the human in-vivo environment. The engineered ETU will reproduce the complexity of cell microenvironment present in-vivo and will be specifically designed to investigate the biophysical barriers affecting drug transport, including convective and diffusive gradients limiting cancer drug perfusion, as identified and predicted by the advanced mathematical models. The ETU will include extracellular matrix and blood vessels models, and host ex-vivo co-cultures of tumor and healthy cells, directly from the patient, allowing to study tumor-stroma interactions. ETU can be also used to evaluate the patient specific response to novel drug carriers.
As targeting moieties for active drug transport through nanocarriers, we developed aptamer ligands for transmembrane receptors overexpressed on the cancer cell surface. Indeed, aptamers have easy synthetic synthesis and modifications to improve their pharmacokinetics (stability and biodistribution) and pharmacodynamics (toxicity and immunogenicity), and show an improved tissue penetration. The functional effects of treatments is experimentally determined by measuring morphological and chemical parameters and will be fed to the mathematical models developed. The output of this analysis will be used to identify the controlling barriers to drug transport and to optimize nanovector design.
In other terms cancer and heathy cell samples taken from the patient can be growth in the ETU. Since ETU mimics the environment originally surrounding the tissue sample, the cancer will develop in vitro following the same trends, as in the human patient. Quantitative analysis of tumor growth, invasion, and response to external stimuli, such as specific controlled therapies, can provide a measurement of key physico-chemical parameters, able to instruct advanced mathematical models. The models can be finally used to predict the response of patient to therapies.
Similar approach is typically applied in the engineering design of complex processes, such as the design of complex industrial plants or complex mechanical equipments, such as a modern, advanced vehicles. In the engineering approach, mathematical models are used to suggest lab-scale experimental models and tests, that can be also a simplified version of the final product, but still contain all the key parameters needed to provide information relevant to the final design of the product. The final result is a non trivial scale-up from lab to full scale.
In the approach we are here proposing the final result will be to design a complex, patient specific, precision therapy.
S. Caserta
Dipartimento di Ingegneria Chimica dei
Materiali e della Produzione Industriale,
Federico II Napoli Italy. CEINGE-Advanced Biotechnology
sergio.caserta@unina.it
S. Guido
Dipartimento di Ingegneria Chimica dei
Materiali e della Produzione Industriale,
Federico II Napoli Italy. CEINGE-Advanced Biotechnology
M. Mugnano
Institute of Applied
Science & Intelligent Systems (ISASI)
“E. Caianiello” – National Research Council, Pozzuoli (NA), Italy
G. Condorelli
Department of Molecular Medicine and
Medical Biotechnology, Federico II
University of Naples, Italy
V. de. Franciscis
IEOS-CNR Italy
V. Cristini
Houston
*Please note: this is a commercial profile