Ann G. Matthysse, Professor of Biology from The University of North Carolina at Chapel Hill, shares her expertise on the transmission of Diarrheagenic E. coli by plants
Diarrheagenic Escherichia coli (DEC) is transmitted to people via the oral route. The bacteria live in the gut of many animals, including cows, deer, and other grazing animals. These animals excrete the bacteria where they may become associated with plant surfaces and be consumed by people. The ingested bacteria multiply in the human intestine and cause illness and diarrhoea. E. coli does not form spores and is not resistant to heat. It is killed by temperatures in excess of 50°C. Thus cooking food before it is eaten kills the bacteria. The principle vehicles on which the bacteria are transmitted to humans are salad and other vegetables that are generally eaten raw and other foods if they are not thoroughly cooked (rare meat and other undercooked foods).
Bacterial adhesion to plant surfaces
- coli actively binds to plant surfaces. The bacteria may bind individually or grow to form a colony or a biofilm on the surface of roots, leaves, or fruits. There may be as many as 105 bacteria per gram on the surface of a contaminated plant. Washing the plant before it is eaten removes only a fraction of the bacteria present. Since many strains of DEC (for example O157:H7) are capable of causing human disease if only 100 bacteria are consumed, the bacteria retained after washing are a significant threat to the health of the consumer.
The mechanism(s) used by the bacteria to bind to plants have been studied extensively with the aim of finding a way to inhibit attachment or to remove attached bacteria efficiently. Substances and structures which appear to be involved in binding to plant surfaces include curli, long filamentous pili, flagellae, outer membrane protein A (OmpA), Cah (calcium dependent adhesin), cellulose, the O antigen, colonic acid, and poly-β-1,6-N-acetyl-glucoseamine [1-3]. In many cases, more than one or two of these are involved in the adherence to plant surfaces and the deletion or removal of one of them has little effect on binding which remains due to the presence of the other adhesins. The role of these adhesins can often be seen by adding the adhesin to a K12 laboratory strain of E. coli that does not bind to the surface in question, for example, alfalfa roots, and observing increased binding of isogenic strains with the added putative adhesin genes. This means that it may be very difficult or impossible to directly inhibit binding of the bacteria to the surface by simply blocking the production of one of these factors. In a few cases, binding can be reduced by ten- or hundred-fold by removing one adhesin such as cellulose or curli, but these effects are generally specific to the bacterial strain and the species or even cultivar of the plant. Thus, they do not provide a general point of attack for DEC removal.
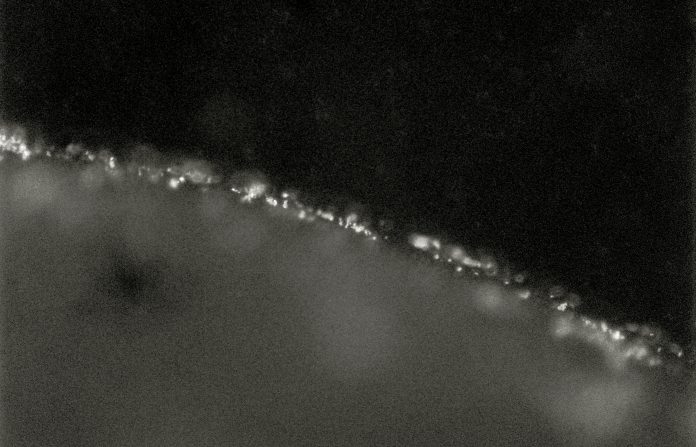
DEC do not bind uniformly to plant surfaces. In general, wound sites are preferred, possibly because they are locations with more available nutrition. On leaves, many strains of DEC show greater binding to the edges of the leaves than to the blade (for an example, see the figure). DEC can also get into stomata where they proliferate. However, they do not appear to invade the leaf tissue from this location. In general, DEC seem to less invasive in plant tissues than Salmonella enterica which should make it easier to kill or remove them.
On plant surfaces which are rough and have crevices, DEC may remain in the crevices after washing [4]. It is unclear if the bacteria are actively bound in these locations or if they are simply trapped in the crevice.
Internalisation of DEC into plants
Although most bacteria are found on the outside of the plant, in some cases DEC have been found to be able to enter the plant and be transmitted from the roots to the shoot or fruit of the plant [5, 6]. The bacteria do not have enzymes to digest the waxy cuticle which covers most of the plant surface, so it is likely that they gain entrance to the plant at wound sites, or through the cracks where lateral roots emerge, or at the region of the root where root hairs are found. There is no cuticle surrounding the root hairs and this is the region where water and inorganic ions are taken up by the roots. This is a particularly vulnerable region for the plant and is in some respects similar to the vulnerability of the human gastrointestinal tract to bacterial infection. When the bacteria invade the root, they do not invade the plant cells. Bacterial colonies were detected between plant cells and inside the cell wall (but not inside the cells) of lettuce leaves [7, 8]. In some cases, bacteria internalised in the root have apparently reached the vascular system and been transported to the leaves and fruits of the plant. The numbers of bacteria found in the leaves after such transport are usually low, less than 103 per gram of tissue.
Removal or killing of internalised bacteria without damaging the quality of fresh produce is very difficult. The only useful strategy to deal with this problem is to prevent internalisation. However, little is known of the conditions which favour DEC internalisation. This topic requires further study to determine if it is possible to reduce the transmission of bacteria via this route.
References
- Jeter, C. and A.G. Matthysse, Characterization of the binding of diarrheagenic strains of E. coli to plant surfaces and the role of curli in the interaction of the bacteria with alfalfa sprouts. Mol. Plant Microbe Interact, 2005. 18: p. 1235-1242.
- Torres, A.G., et al., Differential binding of Escherchia coli O157:H7 to alfalfa, human epithelial cells, and plastic is mediated by a variety of surface structures. Appl. Environ. Microbiol, 2005. 71: p. 8008-8015.
- Shaw, R.K., et al., Flagella mediate attachment of enterotoxigenic Escherichia coli to fresh salad leaves. Environ Microbiol Rep, 2011. 3(1): p. 112-7.
- Wang, H., et al., Effect of surface roughness on retention and removal of Escherichia coli O157:H7 on surfaces of selected fruits. J Food Sci, 2009. 74(1): p. E8-E15.
- Merget, B., et al., Influence of Plant Species, Tissue Type, and Temperature on the Capacity of Shiga-Toxigenic Escherichia coli To Colonize, Grow, and Be Internalized by Plants. Appl Environ Microbiol, 2019. 85(11).
- Eissenberger, K., et al., Adherence factors of enterohemorrhagic Escherichia coli O157:H7 strain Sakai influence its uptake into the roots of Valerianella locusta grown in soil. Food Microbiol, 2018. 76: p. 245-256.
- Seo, K.H. and J.F. Frank, Attachment of Escherichia coli O157:H7 to lettuce leaf surface and bacterial viability in response to chlorine treatment as demonstrated by using confocal scanning laser microscopy. J. Food Prot, 1999. 62(1): p. 3-9.
- Hartmann, R., et al., Internalization of Escherichia coli O157:H7 gfp+ in rocket and Swiss chard baby leaves as affected by abiotic and biotic damage. Lett Appl Microbiol, 2017. 65(1): p. 35-41.
Please note: This is a commercial profile