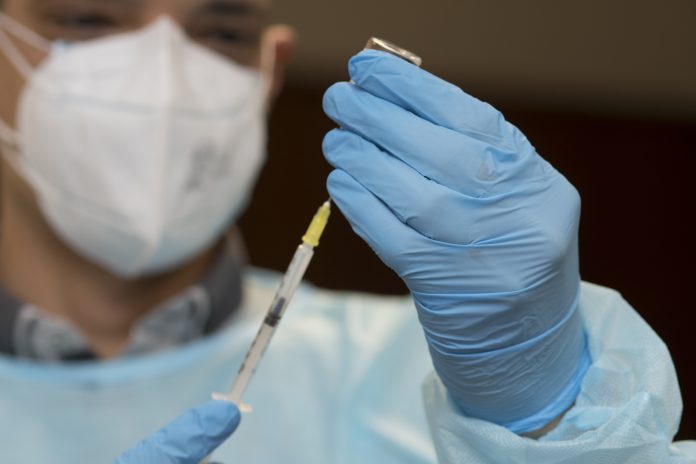
Researchers in the UK started a clinical trial on mixing vaccines in February – today (14 April) a new study will look into mixing Moderna and Novavax doses
The existing clinical trial will now look at mixing the Moderna and Novavax vaccines. The Moderna vaccine is beginning to be used in the UK vaccination programme, while Novavax awaits authorisation.
Mixing vaccines could speed-up vaccination
Scientists now want give a dose of Moderna or Novavax to someone who has already had an AstraZeneca or Pfizer first dose – a move which would significantly streamline the vaccination process.
Novavax has been proven 86% effective against the UK mutation, while Moderna has been proven 94.5% effective in real-world data about healthcare professionals coming out of the US. The UK Government has a contract for 17 million doses of Moderna and 60 million doses of Novavax.
Some countries continue to wait for their first doses, while others are halfway finished with full population innoculation.
The University of Oxford, leading both studies, will recruit adults over the age of 50 who have received their first dose in the past 8 to 12 weeks. A further 1050 people will be brought into the new study.
‘Create resilience within the system’
Matthew Snape, Associate Professor in Paediatrics and Vaccinology at the University of Oxford, and Chief Investigator on the trial said: “If we can show that these mixed schedules generate an immune response that is as good as the standard schedules, and without a significant increase in the vaccine reactions, this will potentially allow more people to complete their COVID-19 immunisation course more rapidly.
“This would also create resilience within the system in the event of a shortfall in availability of any of the vaccines in use.
“We saw a fantastic response to the public from the original COM-COV study, with 830 participants recruited over eight sites in a two-week period in February. We look forward to working with this network and several new sites for the COM-COV2 study.”