The EMA joins the US in approving the 94.1% effective Moderna vaccine for use, while the UK still waits on further regulatory discussions
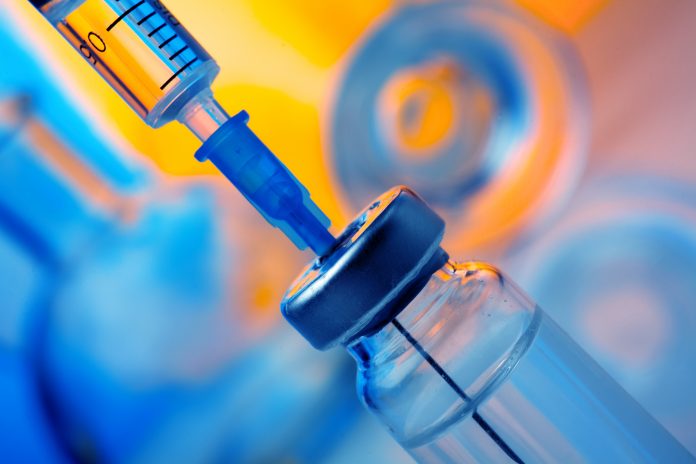
The Moderna vaccine has today (6 January) been approved for use in Europe by the European Medicines Agency (EMA).
The European Commission have bought an initial amount of around 80 million doses for all countries, with the option remaining to buy another 80 million. The question of access to this market of buying vaccinations remains unanswered, with ongoing campaigns to make sure developing nations have equal access to this cure.
However, this does not mean the EU itself has approved the vaccine for use. The regulatory body has suggested approval for the bloc. Health Commissioner Stella Kyriakides tweeted that “authorisation will follow shortly”.
How effective is the Moderna vaccine?
At Phase Three, their trial had 30,000 people, with 15,000 of them taking two real doses and the other half being given empty doses. The real vaccine injections were given four weeks apart, same as the placebo. The first 95 people to develop COVID-19 became the key participants for demonstrating how this new drug works – only five people who took the vaccine contracted COVID-19. Overall, 196 people developed COVID-19 and there was only death. The death was in the group that did not have the vaccine. There were 11 severe cases of the virus among the participants, but this was not in the group that had been given the vaccine.
The trials have been across a spectrum of age, race and ethnicity – with Hispanic, Black, Asian and multiracial participants.
Are there any side effects?
The most common side effects are pain and swelling at the injection site, tiredness, chills, fever, swollen or tender lymph nodes under the arm, headache, muscle and joint pain, nausea and vomiting. The safety and effectiveness of the vaccine will continue to be monitored as it is used across the EU.
These effects usually went away in a few days. However, the company stresses that rarer side effects can only be understood when millions of people are vaccinated. There may not even be any – but this is something about all vaccinations that can only be found out after mass use.
Why is the EU taking so long to vaccinate people?
The European Commission has been criticised for a slower rollout of vaccines than previously expected, approving their first vaccine after the UK and US were well into their vaccination regime.
However, the EMA required a second stage of assessment: a conditional marketing authorisation, which holds pharmaceutical manufacturers to account than the emergency usage given out by the US and UK. Essentially, the UK government will be held responsible for any vaccine mishaps. In the EU, the companies themselves will be responsible.
Speaking to DPA, the EU health commissioner Stella Kyriakides said that the EU planned appropriately, and the shortage of vaccines is an infrastructure problem: “The bottleneck at the moment is not the volume of orders but the worldwide shortage of production capacity.”
The EU has so far signed six contracts – meaning that they have already secured 2 billion doses of the COVID-19 vaccine. They are now waiting for AstraZeneca, Sanofi-GSK, Janssen Pharmaceutica NV, and CureVac.