Contrary to popular belief, OTC drugs are not harmless. Understanding the effects and dangers of OCT abuse and misuse is key to public safety, here UC Davis provides everything you need to know
The term ‘overdose’ refers to the administration, intentional or unintentional, of a drug or other biologically active substance in an amount that exceeds a safe dose and, therefore, can cause toxicity.
Most of us associate this term with use of illicit drugs, such as methamphetamine, or the abuse of regulated, controlled medications, like the opioid fentanyl, likely because adverse effects associated with these drugs are common topics in the news, social media, and movies or books. But any biologically active substance that can be ingested, inhaled, injected, or absorbed through the skin, including compounds essential to life, such as oxygen and water, can cause toxicity when in excess.
Non-controlled, non-prescribed medications, also known as over-the-counter (OTC) drugs, or in the United Kingdom (UK), general sales list (GSL) drugs, represent a large class of biologically active substances that are often under-appreciated as the cause of a significant number of overdosing incidents in humans.
OTC drugs are medications that can be readily purchased without a doctor’s prescription. In certain situations, restrictions are associated with OTC drug use. For example, during the methamphetamine epidemic in the early 2000’s, the U.S. enacted a federal law in 2005 that limited the purchase of medication containing pseudoephedrine, ephedrine or phenylpropanolamine, because these compounds can be used to synthesize methamphetamine.
As another example, in the U.S. state of California, it is currently illegal to sell cough medicine containing dextromethorphan to teenagers younger than 18 years old. The intent of this laws is to limit access of underaged individuals to an OTC medication with high abuse potential: dextromethorphan is an addictive substance that when taken in excess causes effects similar to alcohol. While OTC medications are generally considered safe by regulatory agencies if taken according to the guidelines, they are not without risk, and toxicity caused by overdosing of OTC medications is not uncommon.
OTC drug discovery and development
Significant resources are devoted to the discovery and development of novel medications that are either more effective than existing therapies (for example, new drugs that are better at preventing, slowing the progression or curing disease and/or have reduced adverse effects compared to current treatments) or that treat diseases for which there currently is no effective treatment. The process of drug development from discovery to clinical use follows a formal process illustrated in Figure 1.
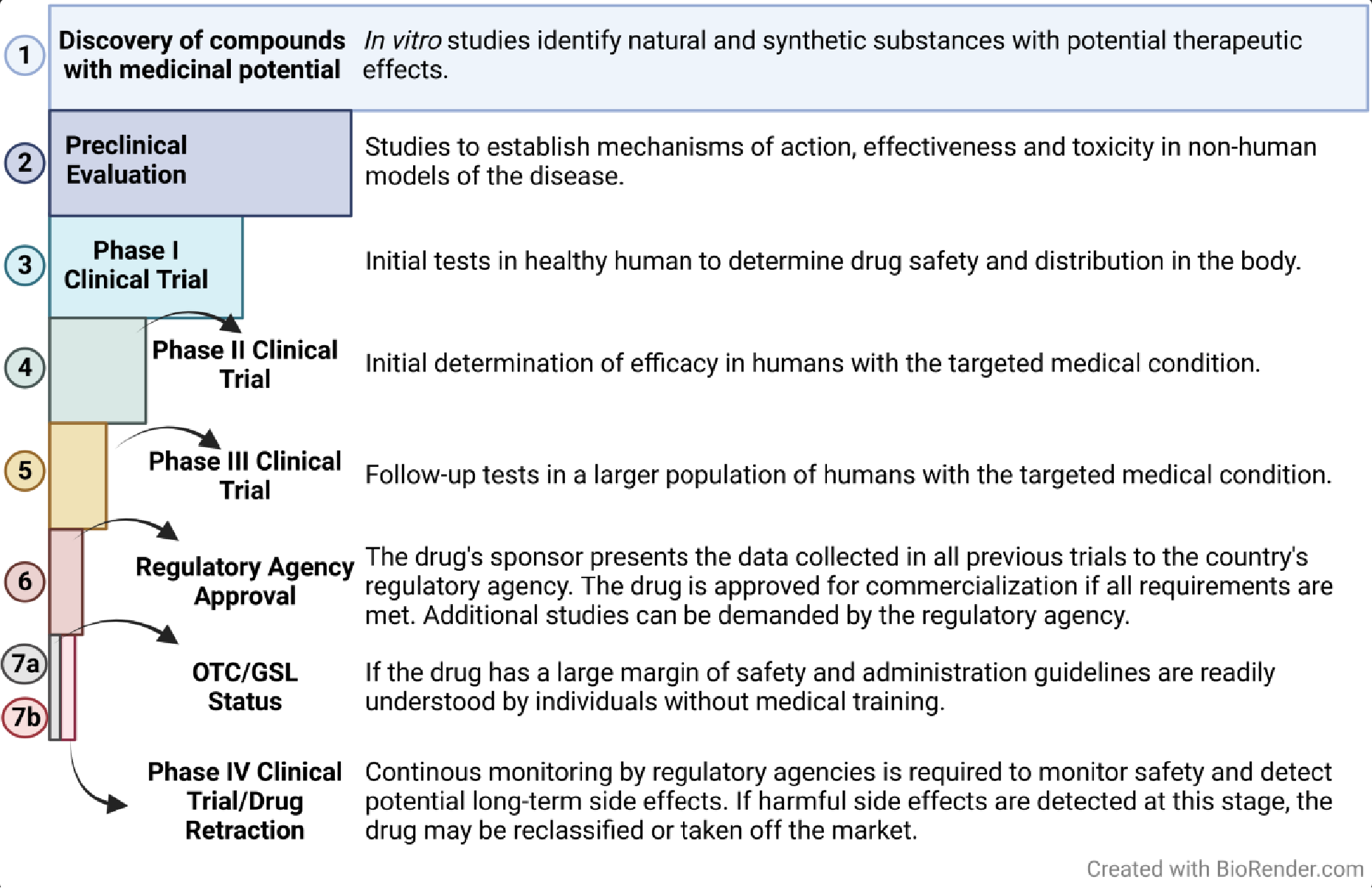
Following the discovery or identification of a compound with potential therapeutic use (these compounds are often referred to as therapeutic candidates), the compound is initially assessed in preclinical studies.
Preclinical evaluation typically relies on experimental animal models of human disease and/or human cell culture models of disease to determine how the candidate drug works. Specific questions addressed in preclinical studies include: (1) what molecule(s) does the drug interact with in the biological system to cause the desired therapeutic effect; (2) how is the candidate drug metabolized; (3) how long does it persist in the body; and (4) are there any adverse or toxic effects under various dosing schedules?
If the preclinical research suggests the candidate drug is effective in treating the disease and has a reasonable margin of safety (the ratio of the dose that has beneficial effects to the dose that causes potential adverse side effects of toxicity is acceptable), the compound advances to Phase I clinical trials in which the safety of the drug is evaluated in a small number of healthy human subjects.
In Phase II clinical trials, the drug is tested for efficacy in a small number of human subjects diagnosed with the disease the drug is meant to treat. If the drug proves efficacious in Phase II clinical testing, compounds proceed to Phase III clinical testing in a larger number of human subjects with the targeted disease to confirm efficacy and safety. Historically, approximately 90% of all new drugs fail during clinical trials and, therefore, do not become available to the public.
Some examples from last year include Amcenestrant for breast cancer, Crenezumab for Alzheimer’s disease, and PF-07304814 for COVID-19. The remaining 10% that pass all three clinical trial phases are then evaluated, prior to being approved for clinical use, by a federal regulatory agency, such as the Food and Drug Administration (FDA) in the U.S. or the Medicines and Healthcare products Regulatory Agency (MHRA) under the supervision of the Department of Health and Social Care in the UK.
The responsible federal regulatory agency of the country (e.g., FDA or MHRA) evaluates all available data regarding the drug’s efficacy and safety to determine whether a new medication can be commercialized. Approval of a drug for clinical use is determined by a number of considerations, including (1) do the drug’s beneficial effects outweigh adverse or toxic risks; (2) how big is the margin of safety; (3) is the labeling adequate; and (4) do production and storage processes guarantee the quality, purity, and strength of the drug?
In the U.S., this information is provided to the FDA by the drug sponsor in the form of a New Drug Application (NDA) that contains all of the data collected from the preclinical and clinical trials. Sometimes the FDA will request additional studies to inform their final decision. Once approved, the medication can be commercialized. A similar process is used by the MHRA in the UK.
Following regulatory agency approval, new medications are classified as either prescription or non-prescription drugs (i.e., OTC/GSL medications). For a new drug to be considered an OTC/GSL, the data available from previous research must prove: (1) it has a high degree of safety; in other words, the drug does not present major health risks for diverse individuals who may use it; (2) compared to prescribed drugs, the risk of misuse or abuse is low; and (3) the therapeutic window (the dose range that produces beneficial effects) either does not cause toxicity or causes only rare minor side effects.
The FDA also requires that the NDA (New Drug Application) have a section demonstrating that potential consumers can understand the information included in the label and can use the medication appropriately without a doctor’s instructions (this is referred to as label comprehension). Regulatory agencies can recommend that the drug sponsor perform complementary studies to assess the ability of consumers to correctly self-select the drug for their condition.
A drug may initially be approved as a prescription only, but the sponsor can apply later for a switch from prescription to nonprescription (Rx-to-OTC switch) if they have acquired sufficient data demonstrating that the drug’s safety and efficacy profile are consistent with OTC labelling. An alternative approach for obtaining the OTC label is to develop a drug following the guidelines provided in an OTC monograph for a given therapeutic indication.

The OTC monograph is basically a “rule book” for a specific therapeutic category that establishes conditions, such as active ingredients, uses (indications), doses, route of administration, labeling, and testing under which an OTC drug is generally recognized as safe and effective.
For example, sunscreens and sleeping pills have OTC monographs that describe what those products may contain and how they should be labeled. Therefore, a sponsor can develop and commercialize a new version of sunscreen or sleeping pills without going through the complex FDA or MHRA approval process if they follow the relevant OTC monograph.
All approved drugs are continuously monitored for side effects while they are being marketed. This is known as the fourth phase of drug development when potential long-term side effects that would be undetectable in the earlier, shorter preclinical and clinical studies may appear. If medications present long-term undesired effects, regulatory agencies can impose interventions, ranging from label adjustments to revoking approval for clinical use.
An example is thalidomide, a drug originally approved for treating pregnancy-induced morning sickness, sleeping problems and anxiety that was subsequently found to cause significant birth defects if taken during pregnancy (approximately 10,000 birth defects in Europe were attributed to the use of thalidomide by pregnant women in the 1960’s). The thalidomide experience resulted in enhanced monitoring and regulation of drug approval and commercialization in several countries.
Nowadays, thalidomide has been repurposed and is approved for the treatment of certain types of cancer, such as myeloma, but not in pregnant women. Other examples include amphetamine and methamphetamine. In the 1930s, these were OTC drugs used to treat colds, hay fever, narcolepsy, and mild depression.
However, abuse and misuse become evident during the same decade as non-medical, recreational use (e.g., mixing in alcoholic drinks) became widespread. In 1959, the U.S. reclassified amphetamine and methamphetamine as ‘prescription only’ drugs. Currently, amphetamines are approved in the treatment of certain sleeping and mental disorders (such as attention deficit and hyperactivity disorder, or ADHD).
The dangers of overdosing on OTC drugs
When used as described in the label instructions, OTCs are generally safe and effective. However, patients presenting with OTC intoxication are not uncommon, as a result of unintentional or intentional OTCs misuse. The most common causes of overdose differ across OTCs (Table 1).
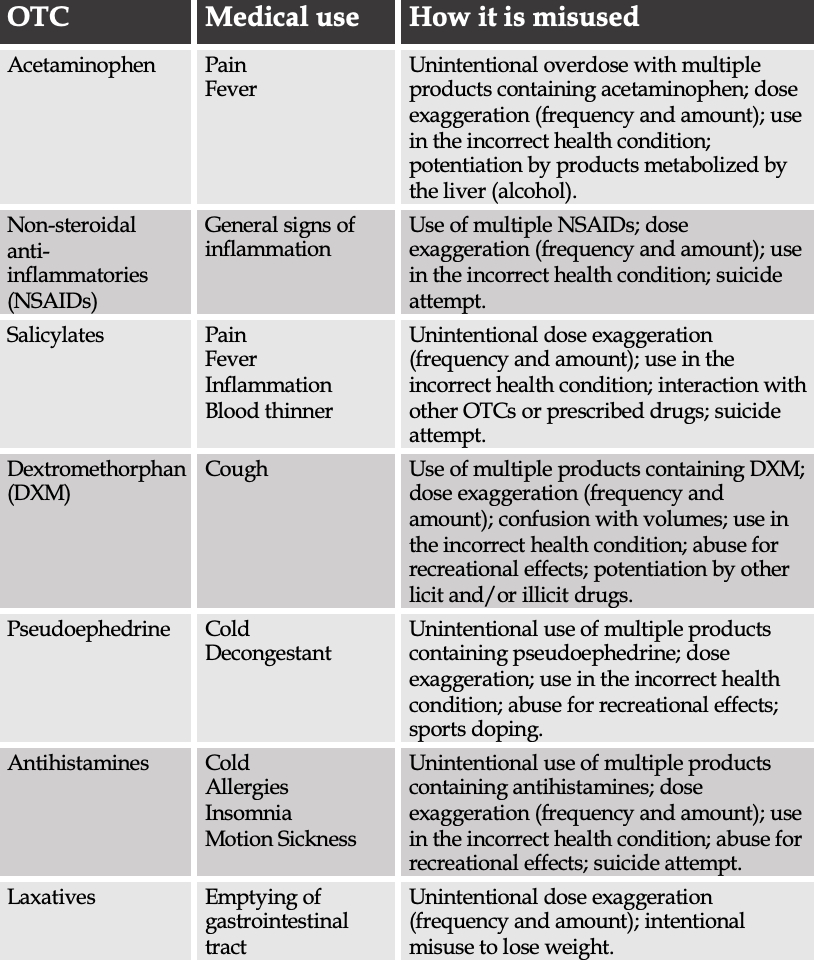
Unintentional OTC misuse
Easy accessibility to OTCs gives the false perception that they are not harmful. As a result, people become careless about the proper way to use these drugs and ignore recommendations printed on the labels.
Lack of information and negligence towards the indications of an OTC can result in accidental misuse that can be life-threatening. Misunderstanding regarding the composition and mechanisms of action of OTCs is fundamental to their unintentional misuse. For example, interactions between OTCs and prescribed drugs can result in dangerous health conditions, such as ‘excessively thin blood’ (excessive bleeding) observed when non-steroidal anti-inflammatory drugs (NSAIDs) or aspirin are taken while using warfarin to prevent blood clotting.
Additionally, unknowingly combining two or more OTCs that have the same mechanism of action increases the risk for adverse side effects. This may happen when people are trying to achieve better and faster results and because they can choose OTCs of the same class marketed with different names, brands, and aesthetics.
A good example is the NSAID ibuprofen, which is sold as Advil, Motrin, Nurofen, and Brufen. Taking multiple OTCs for different concomitant conditions is also dangerous when using drugs that are ‘combination products’, i.e., composed of more than one active ingredient. In those cases, the user may not be aware that they are taking the same compound from different sources and, therefore, the additive doses can exceed safe levels, resulting in adverse side effects.
A well-known example is taking Tylenol, made of acetaminophen, with DayQuil, a product containing acetaminophen, dextromethorphan, and phenylephrine. OTCs can also pose a threat depending on the life stage/health condition of the patient. For example, some OTCs are not recommended to pregnant women, elderly, and newborns or children. To avoid any complications caused by those situations, always be attentive to the product’s label and when in doubt, consult your doctor or pharmacist.
Another situation associated with unintentional OTC misuse is human error and/or confusion. A person may take too high of a dose if they try to (wrongly) increase the expected effectiveness of the drug by taking more than directed or because of confusion about volumes and quantities (e.g., estimating quantities instead of measuring).
The incorrect identification of the conditions affecting one’s health, or even of the medication that should be taken for the condition, are other sources of OTC misuse. Taking anti-inflammatories for coughs or antihistamines for muscle pain is incorrect, but still happens frequently. It is common to observe such mistakes with adolescents since this is the stage of life when they just start to be responsible for the purchase and selection of their own medicines. Once again, to prevent this type of confusion, attention to the product label and asking for help from a pharmacist/doctor are critical.
A well-known example is taking Tylenol, made of acetaminophen, with DayQuil, a product containing acetaminophen, dextromethorphan, and phenylephrine. OTCs can also pose a threat depending on the life stage/health condition of the patient. For example, some OTCs are not recommended to pregnant women, elderly, and newborns or children. To avoid any complications caused by those situations, always be attentive to the product’s label and when in doubt, consult your doctor or pharmacist.
Another situation associated with unintentional OTC misuse is human error and/or confusion. A person may take too high of a dose if they try to (wrongly) increase the expected effectiveness of the drug by taking more than directed or because of confusion about volumes and quantities (e.g., estimating quantities instead of measuring).
The incorrect identification of the conditions affecting one’s health, or even of the medication that should be taken for the condition, are other sources of OTC misuse. Taking anti-inflammatories for coughs or antihistamines for muscle pain is incorrect, but it still happens frequently. It is common to observe such mistakes with adolescents since this is the stage of life when they just start to be responsible for the purchase and selection of their own medicines. Once again, to prevent this type of confusion, attention to the product label and asking for help from a pharmacist/doctor is critical.
The abuse of OTC drugs
Although accidental intoxication with OTCs comprises most cases of OTC overdosing, intentional misuse of OTCs for recreational or non-medical purposes, termed OTC abuse, does occur. The ability to easily obtain OTCs makes them attractive as drugs of abuse, even though some of the desired effects, or ‘highs’, are less potent and/or shorter in duration than can be achieved by abusing controlled substances.
The three most commonly abused OTCs are dextromethorphan, pseudoephedrine, and dimenhydrinate, which are indicated for cough, cold, and motion sickness, respectively. The abuse of such medications can elicit varying sensations, ranging from mild stimulation to hallucination. Unfortunately, the ‘high’ resulting from overdosing on OTC drugs often is accompanied by multiple side effects that can cause toxicity, including death.
The consequences of OTC drug misuse
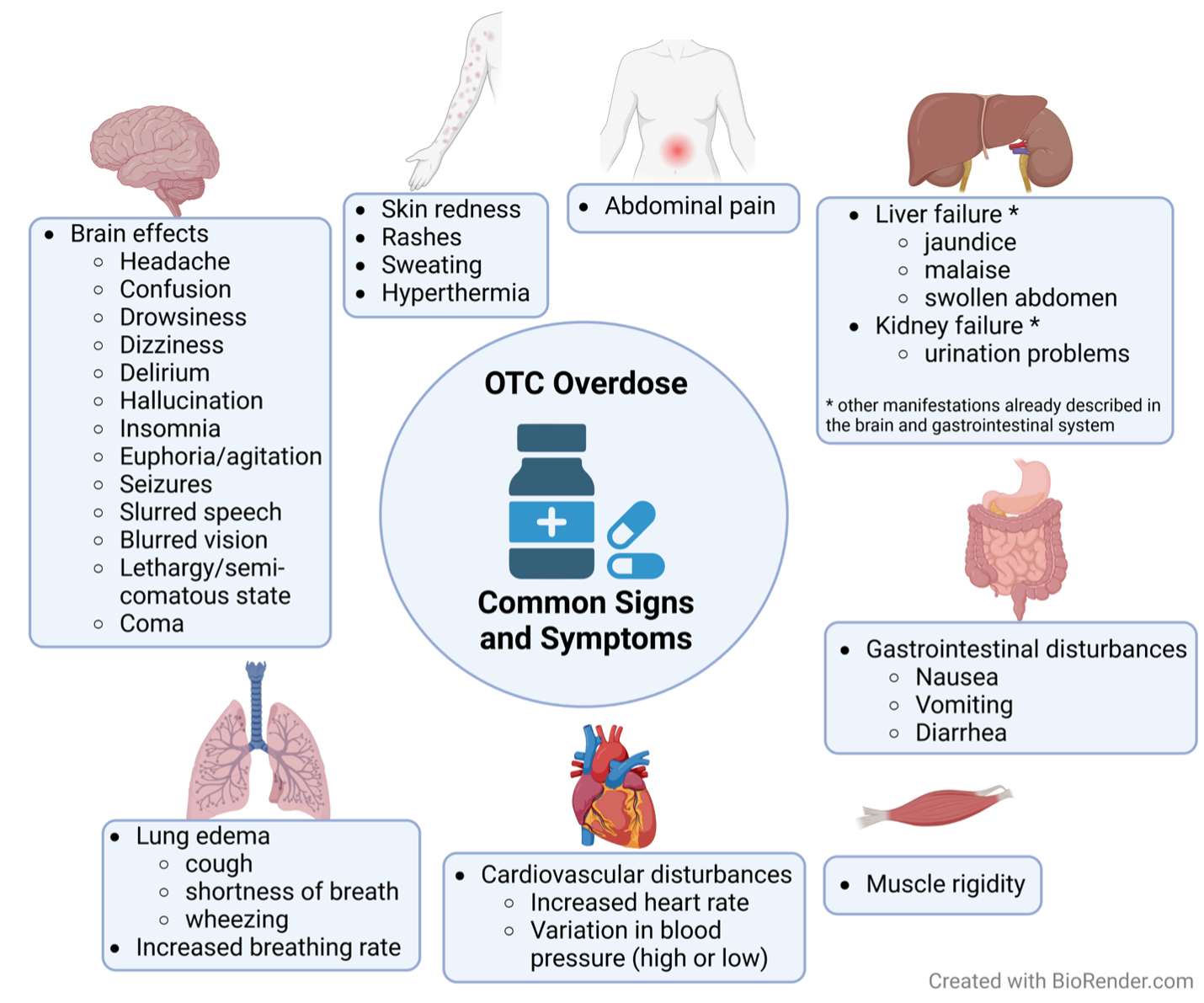
Side effects of OTC overdose vary by OTC (Figure 2), but one common feature of fast-absorbing OTC compounds is acute intoxication. In this scenario, patients may not receive treatment within the optimal time window to prevent morbidities and casualties. Importantly, many of the drugs described here are sold as fast- and slow-release formulations, which directly influence the prognosis, the severity of symptoms and even the likelihood of intoxication.
OTC overdose may be diagnosed by clinical signs and as well as detection of high concentrations of the misused compound in the blood. This diagnosis is assisted by a history of misuse provided by family, friends, medical care workers, or even the patients themselves.
Examining Acetaminophen as an OTC
Tylenol is a formulation of the very well-known pain medication acetaminophen. Acetaminophen is also found in a variety of other products, including cold and cough medications, and acetaminophen overdose is the second leading cause worldwide and the first in the U.S. for liver transplants.
Yearly in the U.S., a total of 56,000 visits to the emergency department are due to acetaminophen intoxication, and this is associated with a mortality rate of approximately 0.9%. Unintentional misuse is often due to daily consumption of too high doses and/or taking a combination of products containing acetaminophen.
While it is safe to use Tylenol at a higher frequency than many other types of drugs, the false certainty that Tylenol is harmless has led many people to assume that precautions are not needed. Close attention should be directed to the minimum time between doses per day – not less than 4-6 h between doses for adults, and not less than 6 h for children – and the maximum dose per day – not to exceed 4 g in adults or 60 mg/kg in kids. Both chronic misuse and acute ingestion of extremely high doses will lead to overdose.
Acetaminophen is metabolized by the liver to form the toxic byproduct N-acetyl-p-benzoquinone imine (NAPQI), which is quickly reduced to nontoxic metabolites excreted by the kidney into urine. However, the metabolism of NAPQI is dependent on glutathione, an antioxidant found in the liver.
During acetaminophen overdose, glutathione can be quickly depleted, resulting in accumulation of the toxic metabolite NAPQI, which eventually causes death of cells in the liver. Treating acetaminophen intoxication is challenging because (1) the first stage, which can last up to 24 hours post-ingestion, is free of symptoms; and (2) administration of the antidote, N-acetylcysteine, which restores glutathione levels, should start within the initial 8 hours of intoxication to improve prognosis.
Gastrointestinal lavage should also be performed within 1 hour post-ingestion, followed by administration of active charcoal. In the absence of these initial therapies, symptoms progress to vomiting, abdominal pain in the region of the liver, and in the most severe cases, renal failure, improper blood coagulation, brain dysfunction, and/or death within 3 days. Patients in advanced stages of acetaminophen intoxication may need liver transplantation. Excessive consumption of alcohol can further deplete glutathione in the liver, thus, heavy drinkers are more susceptible to acetaminophen intoxication at lower doses of the drug than non-alcohol consumers.
Non-steroidal anti-inflammatories (NSAIDs)
NSAIDs are OTC medications commonly used to self-treat moderate headaches, pain and swelling caused by physical trauma and any other physical discomfort caused by inflammation. Unintentional intoxication or attempted suicide with ibuprofen and other NSAIDs are responsible for 103,000 hospitalizations and 16,500 deaths in the U.S. yearly.
No patient should take more than 100 mg/kg of these anti-inflammatories per day, and chronic use should be done with precaution and preferably with medical assistance, especially if the condition that led to the use of such medication persists.
NSAIDs, at high doses, are toxic to the kidneys and the gastrointestinal tract. Injury to these organs can start as early as 4 hours post-intoxication, depending on the dose taken. Initial manifestations are commonly gastroenteric, including nausea, vomiting and abdominal pain.
Headaches and tinnitus can appear, along with dose-dependent rashes, hypertension, convulsions and pulmonary edema. Mental state can be altered and range from mild confusion to delirium and low level of consciousness. To improve prognosis, gastric lavage and active charcoal administration should be initiated during the initial hours post-consumption to limit further absorption of the drug. Supportive treatment to maintain proper breathing, blood circulation, and renal protection are essential to increase survival chances.
Salicylate and its dangers
Widely used for its analgesic and blood thinning effects, aspirin’s active ingredient is acetylsalicylic acid (ASA). Suicide attempts and unintentional ingestion of excessive doses of ASA are emergencies that affect approximately 30,000 people per year. The death (mortality) rate is directly proportional to the severity of intoxication and failure to diagnose and treat the condition during early stages of intoxication.
The severity of salicylate intoxication can be approximated by the concentration of the drug found in the blood: mild symptoms are observed at blood levels around 60 mg/dL; severe signs appear at levels greater than 100 mg/dL. Concomitant use of other OTC or prescribed drugs can contribute to symptom severity, especially if those influence normal breathing and respiration. Chronic use of ASA also induces intoxication with less severe symptoms.
ASA intoxication progresses from nausea, dizziness, vomiting and headaches to confusion, hallucination, and slurred speech, or even seizures, semi-comatose state, and symptoms of brain and lung edema. These symptoms stem from changes to the body’s normal acid-base and electrolyte balances as well as disturbances to the central nervous system. The main goal when treating ASA intoxication is to normalize blood glucose levels, pressure, circulation, and pH, and provide respiratory support. Additional therapies are important depending on the symptom profile. Gastric lavage and use of activated charcoal are indicated if the patient presents shortly after ingestion of the medication, although clinical data has shown no difference in morbidity and mortality when those treatments are given.
Dextromethorphan (DXM), the most abused OTC
DXM is the principal component of cough medications. Currently, DXM is the most abused OTC, despite drug companies trying to combat abuse by creating liquid formulations that require large volumes to produce a ‘high’. In the U.S., approximately 6,000 DXM abusers in search of hallucinogenic and euphoric sensations end up in the emergency department each year seeking help for DXM intoxication.
The maximum recommended daily dose is 120 mg, and occasionally this quantity can be enough to induce euphoria and hallucinations. Importantly, taking higher amounts of DXM can generate severe neurologic symptoms that include seizures, muscle rigidity, and coma. The concomitant use of other legal and illegal stimulants, mainly serotoninergic agents, like cocaine, can increase not only the highs, but also the toxicity of DXM. It should be noted that side effects are seen even with doses considered “safe”.
When DXM is abused, high circulating levels can alter the functioning of some brain neurotransmitters, such as serotonin, causing the previously mentioned neurologic symptoms. DXM also acts outside of the brain, activating the sympathetic nervous system in the periphery to stimulate the ‘fight or flight’ response that manifests clinically as tachycardia (rapid heartbeat), hypertension (high blood pressure), and contracted pupils.
Hyperthermia (increased body temperature) and depressed mental state can also occur following DXM intoxication. Treatment consists of supportive care to maintain normal circulation, breathing, and body temperature. Some cases require sedation of hyper-stimulated individuals, while others demand the use of Naloxone (commonly used for opioid overdose) to treat respiratory and nervous depression.
Activated charcoal can be effective when administered within 1 hour of DXM ingestion. More complicated scenarios can happen due to the combination of DXM with other compounds in cough medication, such as acetaminophen, pseudoephedrine, and antihistamine, making it essential that patients are evaluated for ingestion of other drugs.
Examining Pseudoephedrine
Effective and fast acting, pseudoephedrine is found in many cold medications and decongestants. This drug stimulates the sympathetic nervous system, but is safer than amphetamines because it has limited potential to reach the brain.
However, pseudoephedrine can provoke a sensation of an energy surge, which is one of the reasons it is abused for recreational purposes and to enhance athletic performance.
Because of the latter, it is listed as a doping drug (150 g/mL or higher concentrations in urine is considered a doping violation). Moreover, inattentive use of multiple sources of pseudoephedrine can result in unintentional intoxication, e.g., taking a decongestant, a cold medication, and a rhinitis medication all at once. Recommended daily maximum doses are 240 mg for adults, 120 mg for children 6-12 years old, and 60 mg for children 2-5 years old. Potentiation of the side effects can happen if taken with other medications simultaneously, especially those that induce the same sympathetic stimulation, causing constriction of blood vessels, or even daily stimulant products, such as caffeine and energy drinks.
Symptoms of pseudoephedrine overdose are diverse and can range from stimulant effects like insomnia, agitation, seizures, and hallucinations, to depressive action, like sedation and coma. Other signs of intoxication include headaches, dizziness, altered blood pressure, sweating, nausea and even death. Treatment of intoxicated individuals varies according to the symptoms presented, potentially being very similar to the treatment of DXM intoxication.
Purchases of OTCs containing pseudoephedrine are now regulated (the amount that an individual can buy per unit of time is restricted) as illegal methamphetamine can be easily made from pseudoephedrine-containing medication.
The abuses of Antihistamines
Antihistamines are present in multiple products formulated for colds, allergies, insomnia, and motion sickness. The two main compounds from this group are diphenhydramine (DP) and dimenhydrinate (DM). Both act by inhibiting histamine H1-receptors, with DP having greater anticholinergic properties than DM. Although antihistamine abuse is reported due to their psychedelic and hallucinogenic effects, unintentional intoxication is also common. In addition, antihistamine overdose is used for suicide attempts. Daily use of DP and DM should not exceed 5 mg/kg when taken every 6 hours for children 6 years old and younger, or 400 mg when taken 4-6 times a day for adults.
Antihistamine side effects arise from their anticholinergic action and include redness of the skin, tachycardia, hyperthermia, blurred vision, drowsiness, delirium, hallucinations, seizures, and death. Antihistamine intoxication therapy consists mostly of patient observation and supportive care to manage symptoms. Active charcoal administration within the first couple hours of antihistamine ingestion decreases the severity of intoxication.

Laxatives and OTC abuse
Numerous formulations of laxatives are used to increase gastrointestinal movement and promote emptying of the gastroenteric tract. If those desired effects are maintained for too long, water and nutrient malabsorption can promote life-threatening conditions. Laxative intoxication can be unintentional, but intentional chronic use to lose weight is not uncommon.
In those cases, people can suffer debilitating dehydration and a consequent drop in blood pressure, sometimes accompanied with dangerously low blood glucose levels. Although different formulations can result in distinct symptomatology, common signs of laxative intoxication are nausea, vomiting, diarrhea, bloody stools, abdominal cramps, weakness, dizziness and low blood pressure.
The most severe cases can result in collapsing episodes, coma and death. The fundamental treatment of laxative intoxication consists of maintaining physiological levels of hydration, blood pressure, oxygenation and blood glucose.
The addictive effects of OTCs and further consequences
Not every misuse of OTC leads to addiction, but the OTCs that induce hallucinations, delirium, euphoria, sedation, among other neurologic effects, do have addictive potential. These OTCs happen to be the same ones abused for recreational purposes. These drugs can slowly alter brain chemistry and connectivity, resulting in addiction with subsequent drug tolerance.
Unfortunately, drug tolerance makes abusers more susceptible to the harmful effects of such drugs because, over time, higher and higher doses must be taken to reach the desired effect.
Indiscriminate use of OTCs for their medical effect also has the potential to cause drug addiction and/or drug tolerance, and it is not uncommon for individuals to start using more dangerously potent drugs, both licit and illicit, when they no longer can achieve the ‘highs’ initially produced by OTCs. In addition, people misusing OTCs can experience withdrawal syndrome, characterized by irritability, confusion, anxiety and mood changes.
Conclusions on OCT overdose and the dangers they pose
An important step in reducing OTC overdose is education so people understand that OTCs are not harmless.
This is especially important for adolescents and young adults as this age group seeks OTCs for recreational purposes and is less informed about the proper use of these medications, resulting in a scenario that makes them more prone to overdose from OTCs misuse.
However, intoxication can occur at any stage of life.
People are more likely to avoid situations that cause OTC overdose when they understand it is vital to read drug labels carefully, ask their physician or pharmacist about the correct drugs to take, and/or search online for recommended doses and contraindications of OTC medications.



