Peter H. Santschi, Professor at Texas A&M University at Galveston, TX, shares his expert thoughts on the mobility of iodine species in the environment and solid waste
In order to contain long-lived radioactive iodine (16 Mio year half-life of 129I) from nuclear waste, various methods have been employed, with various success. Iodine-129 is one of three key risk drivers at several U.S. Department of Energy waste management sites. Natural organic matter (NOM) is thought to play important roles in immobilisation of aqueous iodide (I-) and iodate (IO3-) in the environment by forming organo-iodine (org-I), but molecular interactions between NOM and iodine species are still poorly understood.
When appropriate analytical techniques were applied to determine the chemical speciation of iodine in aquatic systems, regardless of surface or groundwater, fresh or ocean water, solution in soils, sediments or cement systems, an almost equal proportion of iodide, iodate and organo-iodine were found (reviewed in Kaplan et al., 2014; Yeager et al., 2017), even in the presence of minimal amounts of natural organic matter (NOM). All three species are always found simultaneously, irrespective of where one measures it: arid, humid, marine systems, U.S. or Japan (Xu et al., 2016).
Therefore, to contain all iodine species at DOE waste sites once they have reached the natural environment, or to effectively remove all radioiodine species from waste streams at the same time remains a challenge. Often, containing at least one of these species, often org-I, is problematic. Organo-iodine species that are found in the environment in the form of humic acids, which are part of the natural organic matter (NOM) matrix, can be either mobile or immobile, depending on conditions.
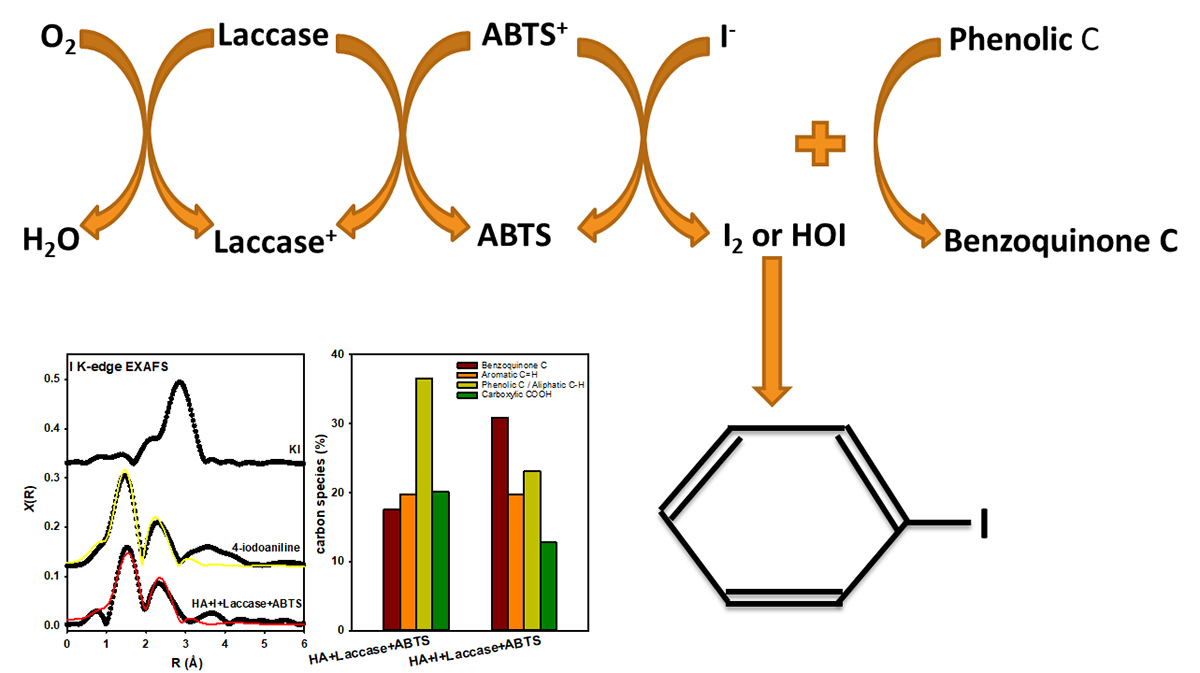
Thus, a better understanding of the molecular binding of the I to the organic core is required to solve this problem. A recent study by Li et al (2019b) aimed to improve our molecular-level understanding of NOM-iodine interactions and enzymatically-mediated iodination reactions in aqueous media. These authors investigated the mechanisms iodine and carbon species are bound to three different humic acid (HA)-I systems using I K-edge XANES and EXAFS and C K-edge XANES spectroscopy: 1) I- in the presence of the laccase enzyme and a mediator (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid, ABTS) at pH 4 buffer, 2) I- in the presence of lactoperoxidase and H2O2 in pH 7 buffer, 3) IO3- at pH 3 groundwater.
Results showed that in both the oxidase and peroxidase systems, I- is oxidized to I2 or hypoiodide (HOI), but the laccase-ABTS mediator system was the most effective at oxidizing I- to I2 or HOI, so that I- uptake by HA increased up to 13.5 mg/g of humic acid. IO3- was abiotically reduced to I2 or HOI. Pathways for HA iodination include covalent modification of aromatic-type rings by I2/HOI or iodine incorporation into newly formed benzoquinone species arising from oxidation of phenolic C species. However, org.-I species can still become mobile in the environment under certain conditions, despite the fact that most of them are contained in the soil. Soil and groundwater contamination are ubiquitous at the U.S. Department of Energy waste management sites and, thus, it is imperative that technologies are developed to contain all iodine species that have already escaped to the environment.
Silver (Ag) based technologies are amongst the most common approaches to removing radioiodine from aqueous waste streams. As a result, a large worldwide inventory of radioactive AgI waste presently exists, which must be stabilised and requires disposal. Recently, Li et al (2019a) examined the efficacy of silver-impregnated granular activated carbon (Ag-GAC) to remove I-, iodate IO3- and org-I from cementitious leachate.
In addition, cementitious materials containing three different aqueous iodine species, I-, IO3-, or org-I loaded Ag-GAC were characterised by iodine K-edge XANES and EXAFS to provide insight into iodine stability and speciation in these waste forms. The results showed that Ag-GAC was very effective at removing I- and org-I, but ineffective for IO3-, from slag-free grout leachate under oxic conditions. I- or org-I removal was due to the formation of insoluble AgI(s) or Ag-org-I on Ag-GAC. When I-loaded Ag-GAC material was cured with slag-free and slag grouts, the I- was released from AgI(s) to form a hydrated I- species.
Conversely, when org-I loaded Ag-GAC material was cured in the two-grout formulations, no change was observed in the iodine speciation, indicating the org-I species remained bound to the Ag. Because little IO3- was bound to the Ag-GAC, it was not detectable in the grout. It was concluded that the grout formulation and iodine speciation in the waste stream can significantly influence the effectiveness of the long-term disposal of radioiodine associated with Ag-GAC in grout waste. All in all, these results show that it is still challenging to contain all radioiodine species in solid waste. This has important implications for estimated risk from nuclear waste disposal.
Please note: This is a commercial profile
References:
Kaplan, D. I., et al. 2014. Radioiodine Biogeochemistry and Prevalence in Groundwater. Crit. Rev. of Env. Sci. & Technol., 44, 2287-2335.
Li, D., et al. 2019a. Radioiodine immobilization of silver-impregnated granular activated carbon secondary solid waste in cementitious systems. J. Env. Radioact., in press.
Li, D., et al. 2019b. Molecular Interaction of Aqueous Iodine Species with Humic Acid Studied by I and C K-edge X-ray Absorption Spectroscopy. ES&T, accepted.
Xu, C., et al. 2016. Factors controlling iodine and 239,240Pu concentrations and mobility in soils from the Northwestern Fukushima Prefecture, Japan. J. Environ. Radioact. 153, 156-166.
Yeager, C.M., et al. 2017. Microbial transformation of iodine: From radioisotopes to iodine deficiency. Adv. Appl. Microbiol., 101, 83-136.











