Researchers from Japanese institutions have revealed insights into the molecular mechanisms underlying autism spectrum disorder (ASD)
The study focused on the role of the KMT2C gene in ASD pathogenesis.
Autism spectrum disorder contains a range of neurodevelopmental conditions characterised by repetitive behaviours and impaired social interactions.
Genetic factors, particularly involving genes related to chromatin modification and gene transcription, have been implicated in ASD development.
The gene KMT2C, containing a key enzyme in histone modification, has appeared as a significant player in autism spectrum disorder susceptibility. Previous studies have linked haploinsufficiency of KMT2C, a condition where one functional copy of the gene is lost, to an increased risk of ASD and other neurodevelopmental disorders.
Mimicking KMT2C haploinsufficiency
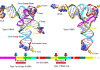
To unravel the impact of KMT2C deficiency, the researchers created a mouse model (Kmt2c+/fs) with a frameshift mutation mimicking KMT2C haploinsufficiency.
Their findings from behavioural analyses revealed that these mice exhibited ASD-related symptoms such as reduced social interaction, inflexibility, auditory hypersensitivity, and cognitive impairments.
Looking deeper into the molecular changes, the team conducted transcriptomic and epigenetic profiling. They observed heightened expression of ASD-associated genes in the mutant mice, compared to the anticipated reduced expression due to KMT2C’s role in gene activation through histone methylation.
The indirect effect on gene expression
Chromatin immunoprecipitation experiments further identified the interaction sites of KMT2C on DNA, indicating an indirect effect on gene expression due to haploinsufficiency.
Single-cell RNA sequencing of newborn mouse brains highlighted the key role of undifferentiated radial glial cells in ASD-related transcriptomic dysregulation.
Potential treatments
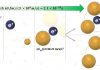
The most impressive discovery was the potential therapeutic intervention using vafidemstat, an inhibitor of histone demethylase.
Treatment with vafidemstat significantly improved social deficits in the mutant mice by restoring the expression levels of dysregulated genes to normal.
This breakthrough challenges the idea that ASD is incurable and shows the promise of targeted pharmacological treatments for neurodevelopmental disorders.
Professor Kato emphasised the potential of drugs like vafidemstat in addressing a spectrum of psychiatric conditions, offering hope for future therapeutic advancements.