Despite the growing burden of mental health problems, there remains a significant unmet medical need. Tadeusz Hawrot, Founder and Executive Director at Parea tells us why new treatments are urgently needed and how psychedelic therapy and medicines can help
The European Union stands at a critical juncture in addressing the escalating crisis of mental health disorders. With the ongoing review of EU pharmaceutical legislation, there is a pressing need to realign our healthcare innovation priorities to better address the unmet needs in mental health care.
The prevalence of poor mental health
Current data paints a concerning picture of the mental health burden in Europe. According to the OECD, half of the population will face mental illness at some point in their lives. (1) In the EU, 32 million citizens (7.2%) suffer from chronic depression, (2) a condition with a high risk of suicide. WHO Europe reports that 75% of major depression cases lack adequate treatment. (3) Up to 50% of treated patients experience treatment resistance, (4) and nearly one-third will attempt suicide. (5) Annually, 130,000 Europeans die by suicide. (6) These stark realities underscore the urgency of addressing mental health as a public health priority.
The unmet need in mental health care
Despite the clear need, the innovation landscape in mental health care is lagging. Current estimates suggest that by 2040, we will only be able to prevent 14% of the burden arising from mental health conditions. (7) The pharmaceutical sector has seen limited progress in developing new treatments for mental health. There are approximately 30 different antidepressants, 20 antipsychotic drugs, seven mood stabilizers for bipolar disorder, and six different classes of drugs for ADHD available. Regrettably, almost none of these are more effective than the medications available three decades ago, despite some improvements in side-effect profiles. (8)
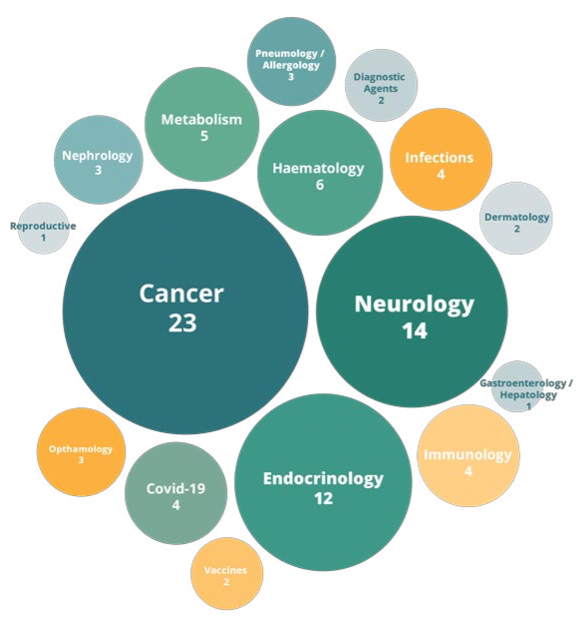
Last year, of the 89 new medicines recommended for approval by the European Medicines Agency, not a single one targeted mental health conditions. (9) Since 2015, only seven neuropsychiatric drugs have been approved globally, in stark contrast to the 80 drugs approved in the field of oncology. (10)
This innovation gap in mental health care is a significant concern. Against this backdrop, among the most promising areas in mental health innovation are novel psychedelic treatments. These therapies have shown potential in clinical trials, offering rapid and durable effects in treating various mental health conditions and substance use disorders. These therapies offer new hope for many patients, particularly those who have not found relief through conventional treatments.
Global landscape for psychedelic therapy
The global landscape for psychedelic therapy is evolving rapidly. This year, Australia became the first country to officially regulate the medical use of psychedelics for the treatment of PTSD and depression. In the US, the first psychedelic therapy is anticipated to be approved by 2024.
Despite significant progress in this field, Europe currently faces a shortfall in late-stage programs that facilitate the approval of new treatments. Numerous early and mid-stage trials are underway within Europe, and the continent has been a fertile ground for pioneering insights in psychedelic research, largely thanks to the contributions of European scientists and study participants. Nevertheless, the majority of the regulatory support and flexibility that encourages the advancement of these treatments into their final stages of development is predominantly seen outside of Europe. This disparity poses a risk of Europe lagging in the international effort to develop and authorize these groundbreaking treatments.
The EU has previously demonstrated its ability to incentivize R&D in specific areas, such as orphan and paediatric medicines. This success can serve as a model for mental health care. By leveraging the pharmaceutical legislation review, the EU has the opportunity to foster innovation and support the development of novel mental health treatments.
To make this possible, the current EU criteria for unmet medical needs need to be expanded from merely looking at life-threatening or severely debilitating conditions to including the prevalence of conditions and their societal impact. This call was included in a recently launched European Parliament policy paper ‘Unmet Medical Needs: Aligning Medical Innovation with Societal Health Needs’ authored by the Psychedelic Access and Research European Alliance (PAREA).
The paper also calls for the establishment of a European Hub for Mental Health R&D. This hub would unite EU institutions, Member States, healthcare funders, and philanthropic organizations to foster evidence-based decision-making in mental health and to set effective incentives and prioritize resources, driving innovation and addressing the substantial unmet medical needs in mental health care across Europe.
The EU is at a crossroads in mental health care. The choices made today in the realm of pharmaceutical legislation will have far-reaching implications for the future of mental health care. It is our collective responsibility to ensure that these choices pave the way for a healthier, more resilient society where mental wellbeing is not just an aspiration but a reality.
References
- OECD (2021), A New Benchmark for Mental Health Systems: Tackling the Social and Economic Costs of Mental Ill-Health, OECD Health Policy Studies, OECD Publishing, Paris
- Eurostat, 2021, 7.2% of people in the EU suffer from chronic depression, viewed 4 October 2023
- United Nations Moldova, 2017, 3 out of 4 people suffering from major depression do not receive adequate treatment, viewed 4 October 2023
- European Medicines Agency 2016, Concept paper on the need for revision of the guideline on clinical investigation of medicinal products in the treatment of depression, European Medicines Agency, viewed 4 October 2023
- Bergfeld IO, et al. Journal of Affective Disorders. 2018;235:362–367; 7. Dong M, et al. Epidemiology and Psychiatric Sciences. 2019;29
- WHO Europe 2022, Statement – First meeting of the WHO/Europe Mental Health Coalition – From debate to action, viewed 4 October 2023
- Remes, J. et al. Prioritizing health: a prescription for prosperity. McKinsey 2020, viewed 18 October 2023
- Insel, T., 2022. Healing: The Path from Mental Illness to Mental Health
- European Medicines Agency 2022, Human Medicines Highlights 2022, viewed 8 June 2023
- Fortune, 2022. Psychedelic startups want to solve the mental health crisis , viewed 8 June 2023
Contributor Details
Editor's Recommended Articles
-
Must Read >> Breaking the stigma: Men’s mental health matters