Kate Fry, CEO of America’s Blood Centers, discusses the essential role of blood products and transfusion medicine in the U.S. and the efforts to ensure blood’s safety and availability
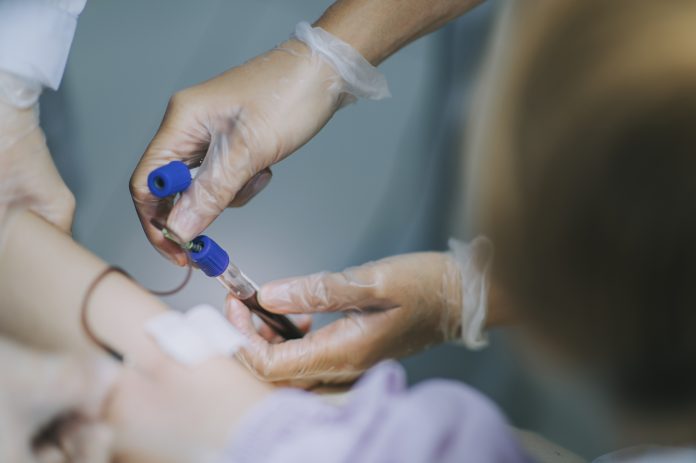
Blood is recognised around the world as an essential medicine. In the United States, blood transfusions remain one of the most common hospital procedures, with a patient requiring a blood transfusion every two seconds.
The collection of blood and recruitment of blood donors relies on a network of not-for-profit blood center located throughout the country.
This model contrasts the U.S. with many other developed nations that rely on a nationalised blood system or a single primary blood supplier.
Individuals can locate their nearest community blood center at www.americasblood.org.
The need for more blood
The US blood supply remains dependent on a voluntary, non-renumerated base of blood donors. These donors are essential to surgeries, and organ and bone marrow transplants.
Despite the importance and constant need for blood, only 3% of Americans donate blood yearly. This percentage continues to decline, with the most acute declines occurring in younger generations.
The rate of younger donors who donated blood – those between the ages of 16 and 24 – decreased by double digits from 2017 to 2019 alone.
The diversity of the U.S. donor base also remains a concern, with less than 20% of all blood donations coming from communities of colour.
A wide variety of conditions that disproportionately impact minority populations require ongoing blood transfusions as part of a patient’s care management, and these patients need blood that is closely matched beyond just blood type.
This type of closely matched blood is most likely from someone of a similar racial and ethnic background.
Regulation of the blood supply
The U.S. Food and Drug Administration (FDA) licenses and regulates blood centers.
Within the FDA, the Center for Biologics Evaluation and Research (CBER) regulates the collection of blood and blood components used for transfusion or manufacturing pharmaceuticals derived from blood and blood components.
CBER regularly publishes guidelines and regulations promoting the nation’s blood supply’s safety, purity, and potency.
These may include testing requirements for infectious diseases, safety requirements for the donation process, and/or donor eligibility requirements.
As a result of the multi-layered system of safety standards, the U.S. blood supply is the safest it has ever been.
During the recent COVID-19 pandemic, the FDA and CBER worked with the blood community to ensure the blood supply’s safety while recognising the pandemic’s impact on blood collection efforts.
The FDA’s efforts included amending blood donor eligibility requirements that safely increased the number of eligible donors and changing regulations without impacting safety.
The FDA was also extensively involved in regulating the use of COVID-19 Convalescent Plasma (CCP), with more than 500,000 units of CCP collected by U.S. blood establishments.
Public policy priorities
As the blood community looks forward to its work with the FDA, Congress, and other government partners, two areas remain at the forefront: expanding the pool of blood donors and available blood through awareness and education efforts and ensuring blood transfusions are available to patients when and where they need blood.
Over the past year, the FDA has relied on extensive data and science to make fundamental changes to blood donor eligibly, including the re-entry of previously deferred donors from the potential risk of vCJD based on time spent in specific European nations and a move to individual donor assessment, which allows for some previously deferred gay or bisexual men, and women who have sex with bisexual men, to donate now.
The blood community supported these changes to safely allow more individuals to contribute and treat all donors with the fairness, equality, and respect they deserve.
The U.S. blood community is also actively promoting access to blood products in emergency settings outside of the traditional hospital environment.
Studies have demonstrated that blood transfusions following trauma save lives. One study found up to 5% increased odds of mortality for every minute of delay in access to blood.
According to the U.S. Centers for Disease Control and Prevention (CDC), unintentional injury is the leading cause of death among individuals ages 1-44.
Bleeding is the leading cause of preventable death following a severe injury, and sadly, up to 56% of those victims die before ever reaching the hospital.
Yet, due to local, state, and federal policies, most Americans cannot access blood before reaching the hospital.
Blood centers’ expanding mission
While challenges remain, blood collection and transfusion medicine opportunities are also expanding their mission to positively impact even more lives through advancements in biotherapies.
Community blood centers have existing infrastructure and clinical expertise to make an immediate impact in this quickly evolving area of healthcare with the appropriate recognition and resources.
Overall, the blood community remains an essential part of the nation’s healthcare system, dedicated to saving and improving lives no matter where and when it is required. America’s Blood Centers urges policymakers, regulators, community partners, businesses, civic organisations, and individuals to commit to supporting the blood supply to ensure blood remains safe and available to every patient in need.